Bonami Laboratory
Division of Rheumatology and Immunology
Department of Medicine
Research Focus
Our overarching goal is to identify mechanisms by which autoantigen-specific B lymphocytes breach immune tolerance barriers to drive autoimmune disease.
Research Program
B lymphocytes in autoimmune disease: Goals. B lymphocytes are immune cells that attack pathogens like flu. Under the wrong circumstances, they can also target "self" proteins to cause autoimmune diseases. Autoantibodies signal this attack is happening, sometimes well before disease symptoms appear. Autoantibodies may directly cause tissue destruction, or they may simply serve as red flags of inappropriate autoreactive T cell-B cell interactions. Our primary research interest is to understand how self-reactive B lymphocytes expand in the repertoire to drive autoimmune disease. Approaches. We combine wet lab and computational approaches along with unique human biospecimen respositories and mouse models to increase our understanding of how autoimmunity arises in the B cell compartment in several autoimmune diseases.
Type 1 diabetes: Several autoantigens (self proteins) are targeted in type 1 diabetes, including insulin. Selective elimination of anti-insulin B lymphocytes prevents type 1 diabetes in a mouse model, highlighting this population of cells as an attractive therapeutic target. We are currently employing high-throughput approaches to interrogate this critical population of autoantigen-presenting cells in type 1 diabetes patients who are in the very early, pre-symptomatic stages of disease. These studies are being performed in collaboration with Daniel Moore, M.D., Ph.D. at Vanderbilt which serves as a T1D TrialNet center. Our long-term goal is to use innovative approaches to study and target this dangerous population of immune cells to hasten development of safe and effective therapies for this devastating disease.
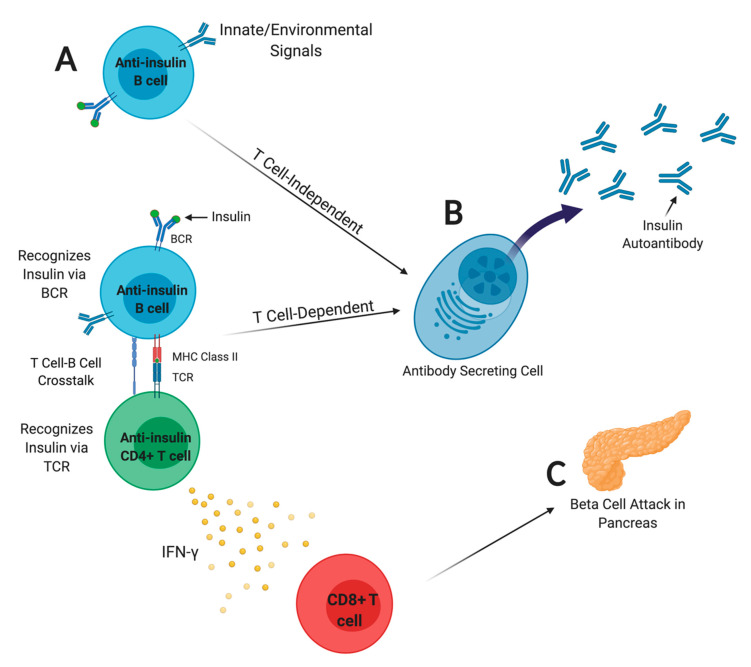
FIGURE: B lymphocytes present insulin autoantigen to drive beta cell attack. A) Anti-insulin B lymphocytes internalize insulin autoantigen via the B cell receptor (BCR) and present insulin peptide to T cells. B) Anti-insulin B lymphocytes may differentiate to become insulin autoantibody-secreting cells, which is a major T1D biomarker used to predict disease risk. Autoantibody does not directly mediate beta cell destruction; rather, it signals T-B cell interaction. C) CD4 T cells produce inflammatory cytokines that promote CD8 T cell attack of beta cells. Figure from Felton, Conway, and Bonami, Biomedicines, 2021. PMC7824835, created with BioRender.
Myositis: The myositis spectrum includes several clinical disease phenotypes. Histidyl tRNA anti-synthetase syndrome (HARS) is defined by autoantibodies that recognize this autoantigen, also called Jo-1. We are currently collaborating Rheumatology/Immunology Division clinical faculty, including Leslie Crofford, M.D. and Erin Wilfong, M.D., Ph.D. to investigate Jo-1-binding B cells using approaches outlined above to better understand how they breach immune tolerance to expand and differentiate to secrete Jo-1 autoantibody used clinically to diagnose HARS. We aim to develop new biomarkers and identify new pathways to target to selectively impair autoreactive B cell function as a means to limit disease progression and track favorable responses to new immune therapies in clinical trials.
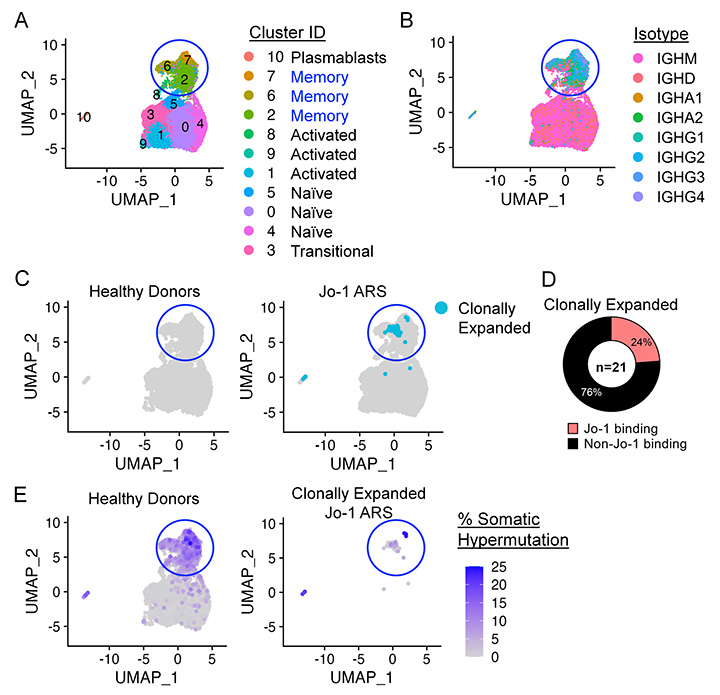
FIGURE: Phenotypic, transcriptional, and B cell receptor (BCR) repertoire profiling of patients with rheumatic disease. A-B) Single-cell transcriptomic (RNA-seq) and BCR repertoire (BCR-seq) profiling (10X Genomics) is shown for Jo-1 autoantibody-positive anti-synthetase syndrome (Jo-1 ARS) patients and healthy controls. 24% of clonally expanded B cell clones (C) were found to bind Jo-1 autoantigen (D), which varied in their extent of IgH somatic hypermutation (E).