Research Projects
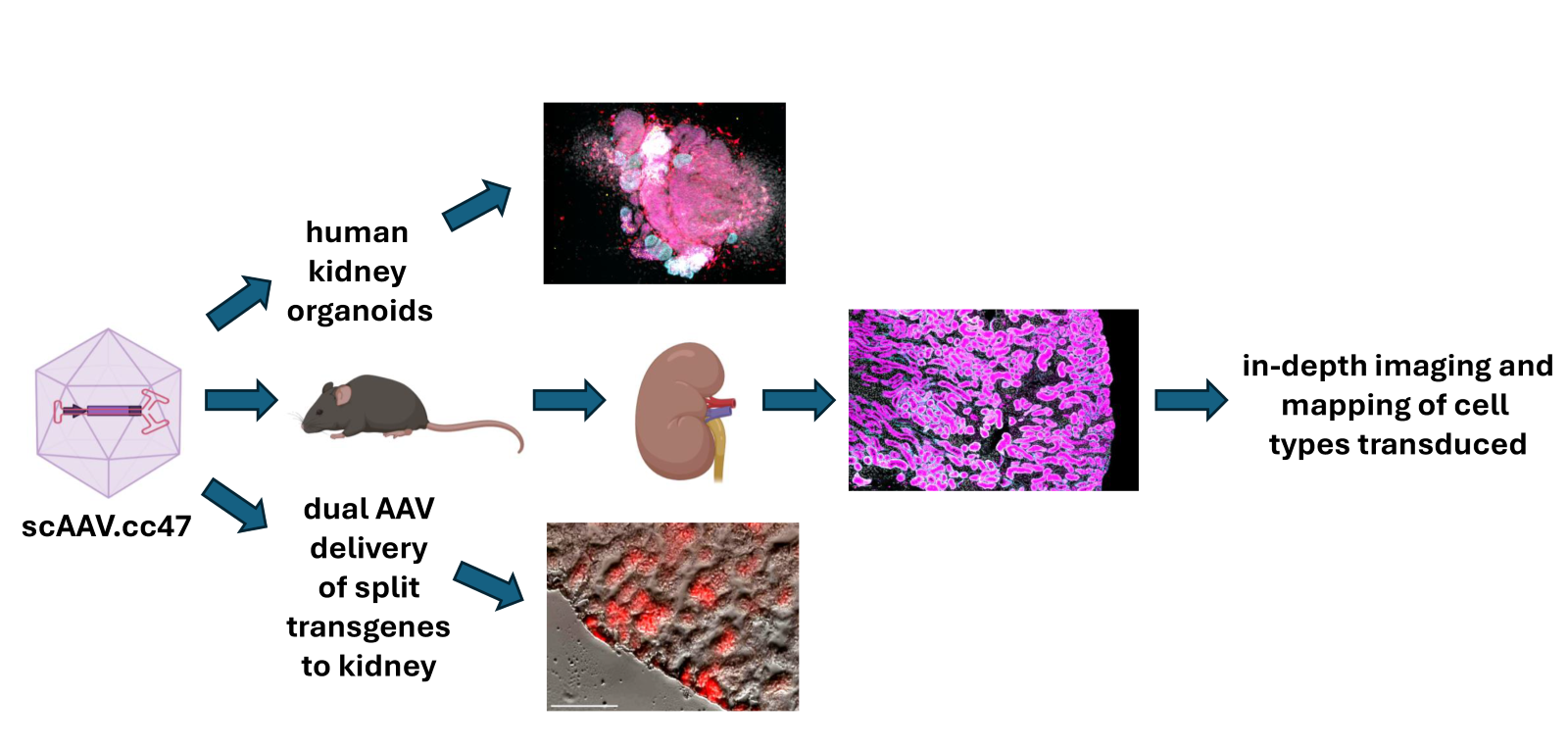
Efficient kidney gene transfer and proximal tubule transduction using self-complementary AAV.cc47 vectors
Gene delivery to critical cell types within the kidney can enable preclinical evaluation of gene therapies for kidney disease. The novel adeno-associated virus AAV.cc47 was discovered after sequential evolution in mice, pigs and macaques, and improved transduction in multiple tissues but without in-depth exploration of the kidney.
We observed robust kidney transduction by AAV.cc47 vectors in mice in vivo and in human kidney organoids compared to AAV9, mostly within the proximal tubule (PT) epithelium. We then developed a quantitative analysis method of transgene expression utilizing automated classification of nephron cell types coupled with cellular expression. Despite exhibiting similar biodistribution to AAV9 in renal and extrarenal tissues, AAV.cc47 consistently transduced the kidney at higher efficiency, with >80% of PT epithelium transduced at low, systemically administered vector dose. Self-complementary AAV.cc47 vectors appear to transduce a subset of PT epithelium, with undetectable transduction of non-PT cells.
This method could be adapted to evaluate different AAV vectors transducing other kidney cell types. We also demonstrate the utility of dual AAV.cc47 vectors to increase genome payload capacity for kidney gene transfer. AAV.cc47 represents a promising vector for use as a research tool and possibly clinical application for kidney disease.
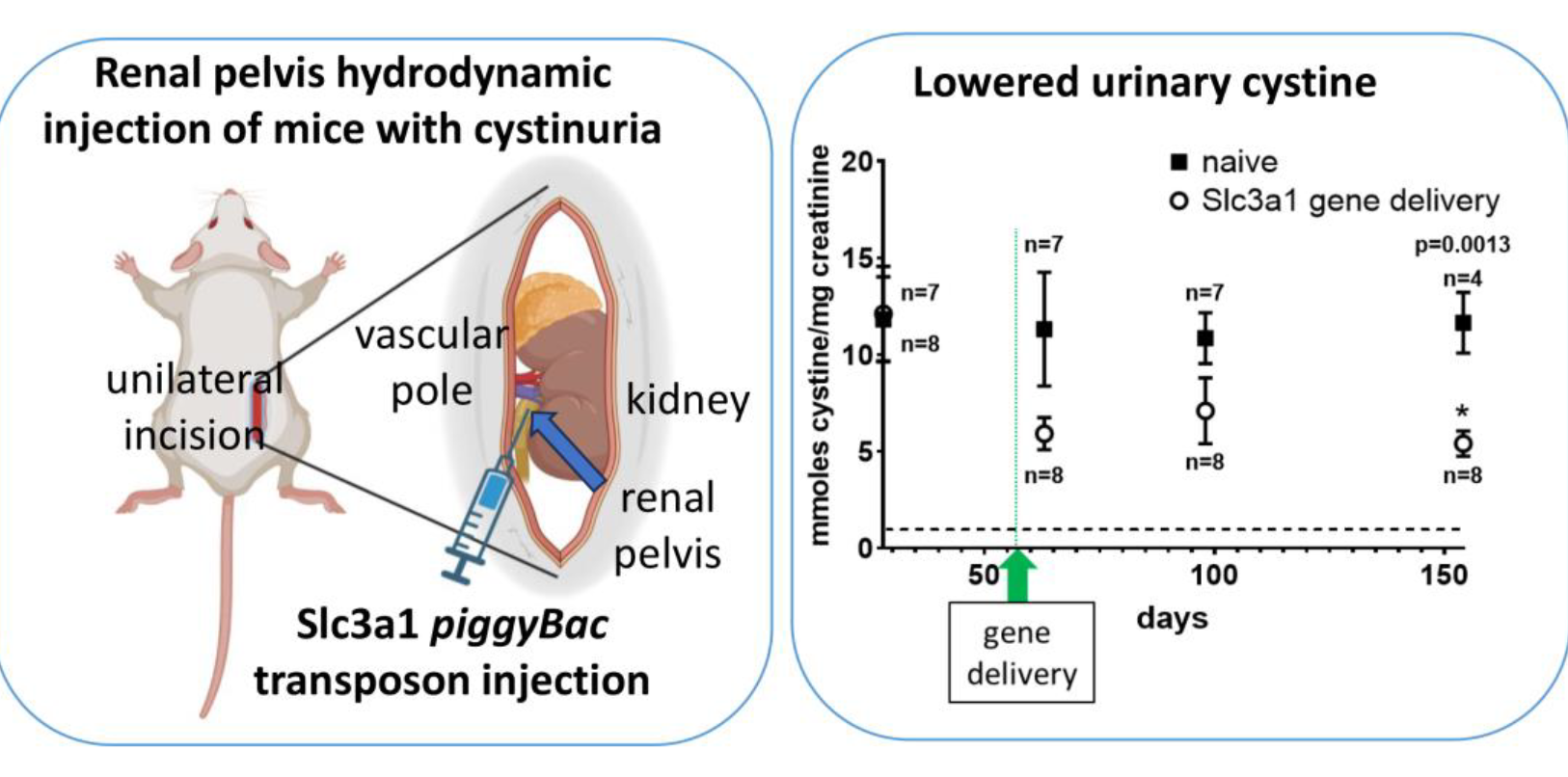
Partial correction of cystinuria type A in mice via kidney-targeted transposon delivery
We used kidney-targeted, non-viral, transposon-mediated gene delivery to express the mouse Slc3a1 transgene in one kidney of cystinuria type A (Slc3a1 -/-) mice. We found a 44% reduction in urinary cystine concentration at 154 days post-gene transfer, although there was no significant effect on cystine stone formation.
Our results indicate that it is possible to achieve kidney-targeted gene transfer, resulting in reduction of cystine concentration in the urine of a cystinuria type A animal model. This proof of concept lays the foundation for future studies directed at gene therapy for cystinuria and other kidney diseases.
Evolving adeno-associated viruses for gene transfer to the kidney via cross-species cycling of capsid libraries
The difficulty of delivering genes to the kidney has limited the translation of genetic medicines, particularly for the more than 10% of the global population with chronic kidney disease. Here we show that new variants of adeno-associated viruses (AAVs) displaying robust and widespread transduction in the kidneys of mice, pigs and non-human-primates can be obtained by evolving capsid libraries via cross-species cycling in different kidney models. Specifically, the new variants, AAV.k13 and AAV.k20, were enriched from the libraries following sequential intravenous cycling through mouse and pig kidneys, ex vivo cycling in human organoid cultures, and ex vivo machine perfusion in isolated kidneys from rhesus macaques.
The two variants transduced murine kidneys following intravenous administration, with selective tropism for proximal tubules, and led to markedly higher transgene expression than parental AAV9 vectors in proximal tubule epithelial cells within human organoid cultures and in autotransplanted pig kidneys. Following ureteral delivery, AAV.k20 efficiently transduced kidneys in pigs and macaques. The AAV.k13 and AAV.k20 variants are promising vectors for therapeutic gene-transfer applications in kidney diseases and transplantation.
Gene and cell therapy for kidney disease
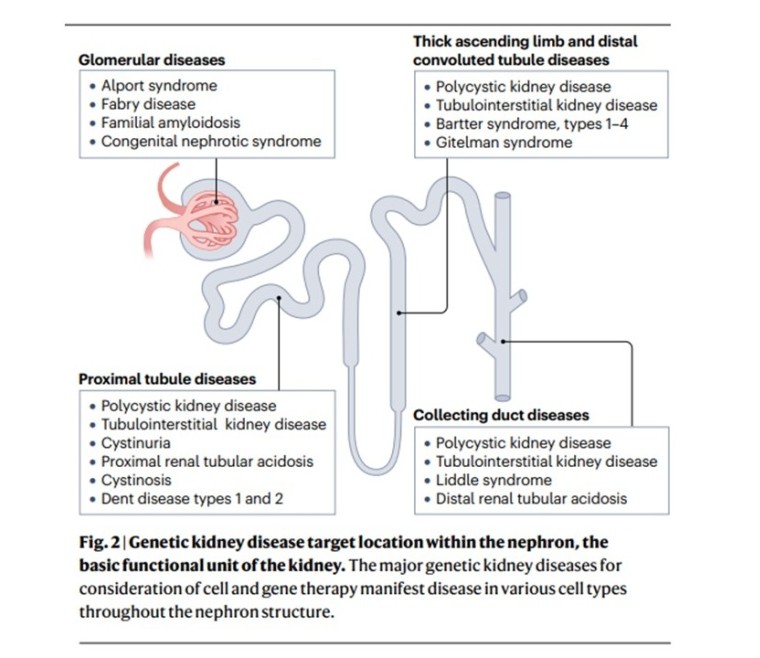
Kidney disease is a leading cause of morbidity and mortality across the globe. Current interventions for kidney disease include dialysis and renal transplantation, which have limited eficacy or availability and are often associated with complications such as cardiovascular disease and immunosuppression. There is therefore a pressing need for novel therapies for kidney disease. Notably, as many as 30% of kidney disease cases are caused by monogenic disease and are thus potentially amenable to genetic medicine, such as cell and gene therapy.
Systemic disease that affects the kidney, such as diabetes and hypertension, might also be targetable by cell and gene therapy. However, although there are now several approved gene and cell therapies for inherited diseases that affect other organs, none targets the kidney. Promising recent advances in cell and gene therapy have been made, including in the kidney research field, suggesting that this form of therapy might represent a potential solution for kidney disease in the future.
In this Review, we describe the potential for cell and gene therapy in treating kidney disease, focusing on recent genetic studies, key advances and emerging technologies, and we describe several crucial considerations for renal genetic and cell therapies.
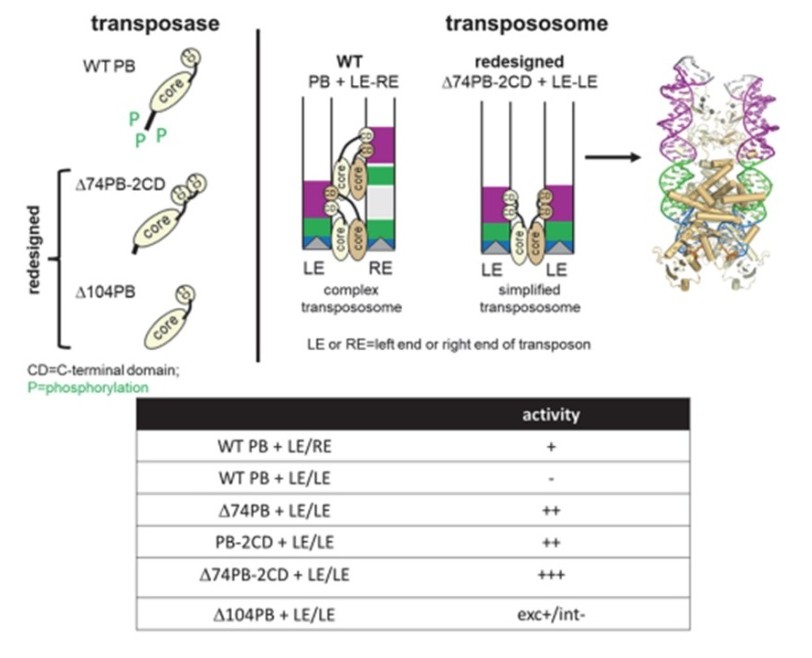
Transposase N-terminal phosphorylation and asymmetric transposon ends inhibit piggyBac transposition in mammalian cells
DNA transposon systems are widely used in mammalian cells for genetic modification experiments, but their regulation remains poorly understood. We used biochemical and cell-based assays together with AlphaFold modeling and rational protein re-design to evaluate aspects of piggyBac transposition including the previously unexplained role of the transposase N-terminus and the need for asymmetric transposon ends for cellular activity.
We found that phosphorylation at predicted casein kinase II sites in the transposase N-terminus inhibits transposition, most likely by preventing transposase–DNA interactions. Deletion of the region containing these sites releases inhibition thereby enhancing activity. We also found that the N-terminal domain promotes transposase dimerization in the absence of transposon DNA. When the N-terminus is deleted, the transposase gains the ability to carry out transposition using symmetric transposon left ends. This novel activity is also conferred by appending a second C-terminal domain. When combined, these modifications together result in a transposase that is highly active when symmetric transposon ends are used.
Our results demonstrate that transposase N-terminal phosphorylation and the requirement for asymmetric transposon ends both negatively regulate piggyBac transposition in mammalian cells. These novel insights into the mechanism and structure of the piggyBac transposase expand its potential use for genomic applications.