Overview
The Roden laboratory’s research is focused on mechanisms underlying variability in response to drug therapy, with a particular focus on therapies used to treat cardiac arrhythmias. The individual projects focus on emerging models in molecular medicine and genetics and incorporate the expertise of the laboratory in electrophysiologic methods.
Discovery
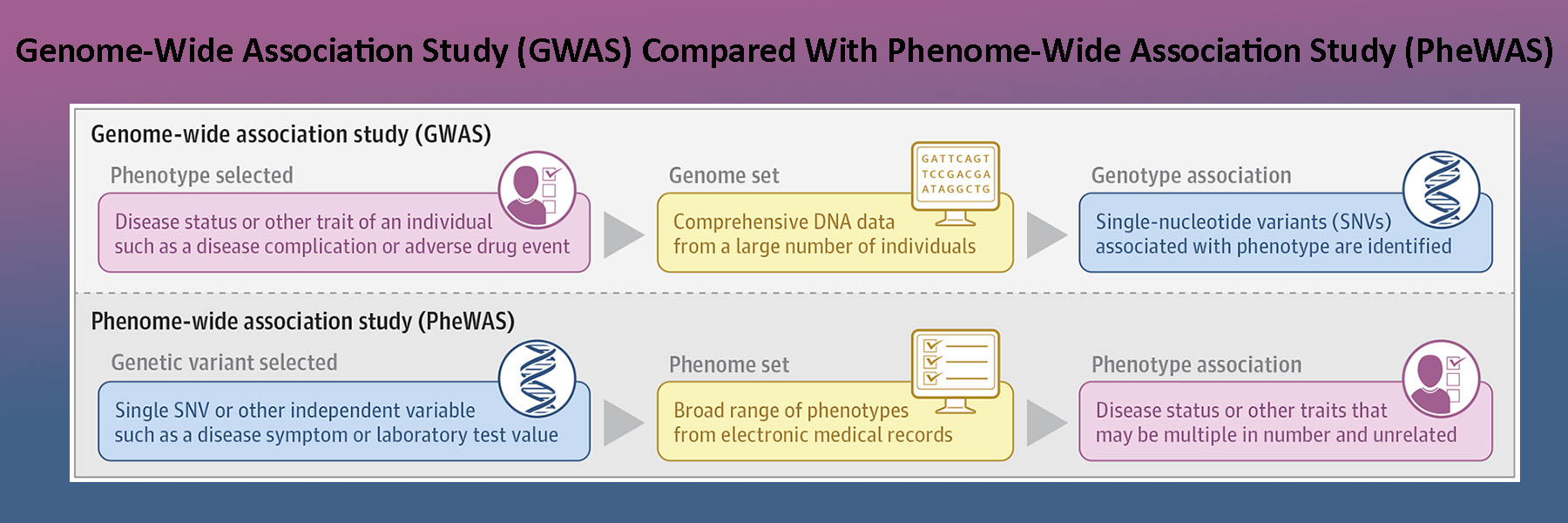
The Roden Lab has a strong tradition in translating discovery from the bedside to the bench and back. Thus, another focus of current work is studies of arrhythmia genomics and pharmacogenomics. Dr. Dan Roden directs the Improving Prediction of Drug Action (IPoDA) program, the Vanderbilt site as part of the NIH Pharmacogenetics Research Network (PGRN) and A Novel Molecular Target in Atrial Fibrillation program, the Vanderbilt site of the American Heart Association’s (AHA) Strategically Focused Atrial Fibrillation Research Network (AF-SFRN).
AHA AF-SFRN / A Novel Molecular Target in AF
One-third of human adults will develop atrial fibrillation (AF) in their lifetimes. Atrial Fibrillation causes burdensome symptoms, stroke, and increased mortality, but for the past 20 years we have not had truly important changes in treatment or prognosis. We need new approaches. Many factors, such as aging, diabetes, high blood pressure, or having an affected family member, increase AF risk. But not everyone with AF has all these risk factors and some have none.
The Roden has discovered specific molecules generated by inflammation that cause atrial tissue damage and increase AF risk. Our Center will build on these findings to test how inflammation contributes to AF risk across all patients, and test a new therapy designed to prevent the tissue damage leading to AF.
Population Project (Roden lab): Identifying AF subtypes driven by inflammation: While atrial fibrillation is a very common problem, it comes in many 'flavors': some patients have incapacitating symptoms and others have none. Some patients have many recognized risk factors, others have none. Some patients respond well to certain drugs, others don't.
Our goal in the Population Project is to use advanced computer-based techniques to identify subgroups of patients with AF in whom specific risk factors are causing AF, or in whom specific treatments 'like the anti-inflammatory approaches being developed in other Projects at this Center may be especially effective. In this work, we will build a large collection of patients with and without AF so other groups can work with us to answer other questions related to AF, its treatment, and its outcomes.
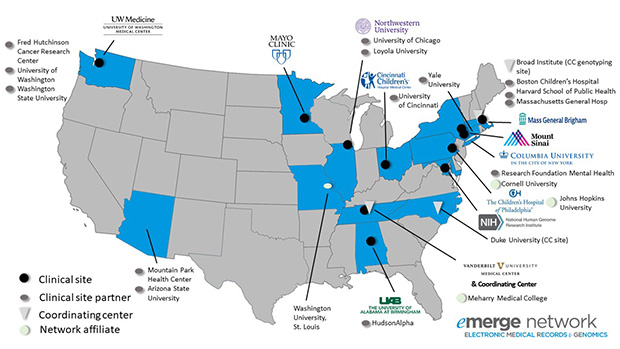
NIH PGRN / IPoDA
It is a given in clinical medicine that patients vary in their responses to drug therapy: lack of efficacy is common and serious adverse drug reactions (ADRs) remain a leading cause of morbidity and mortality. In our Center, three interlinked projects are targeted to improve the prediction of drug action, with the aim of improving current approaches to individual patient therapeutics and drug development.
The project focuses on:
- A new paradigm for identifying patients and drugs at risk for QT prolongation
- Understanding and preventing HLA-associated drug reactions
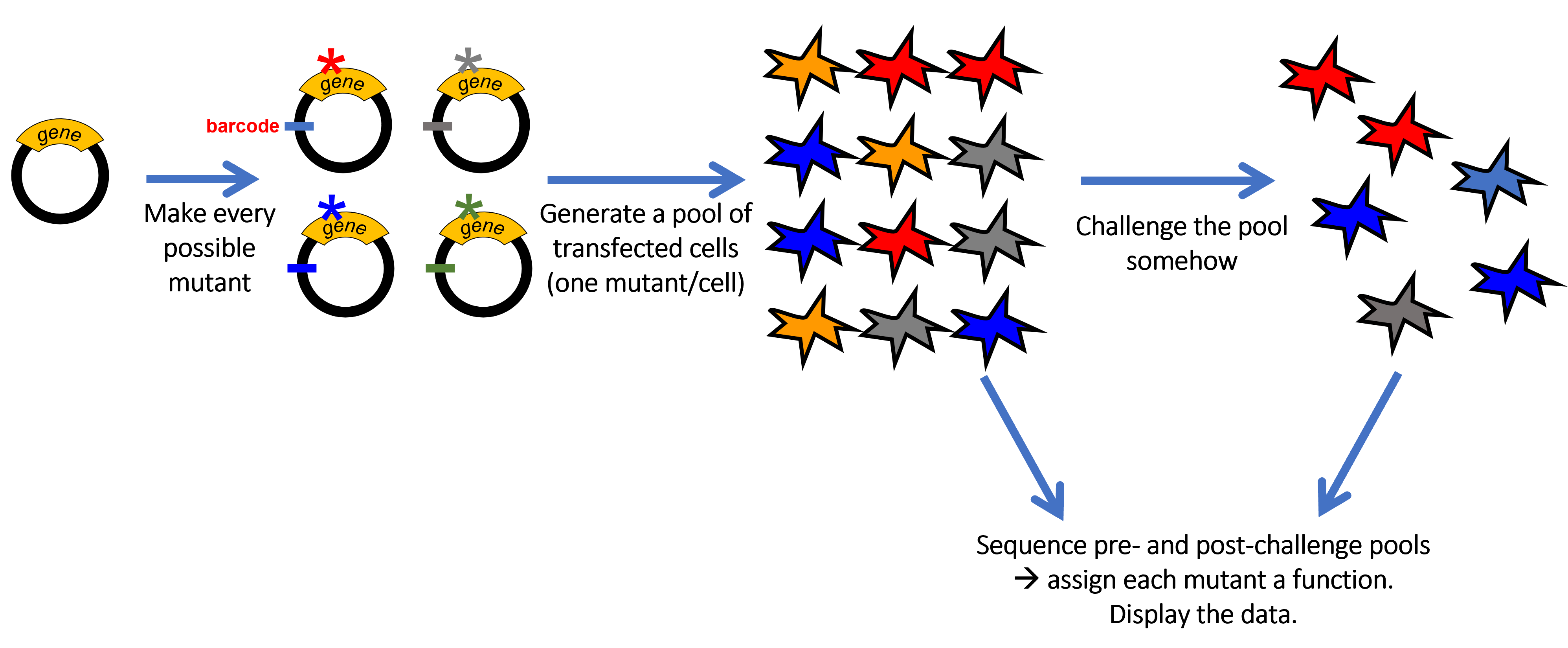
Precision phenomics for personalized drug therapy Project 1 (Roden lab): A new paradigm for identifying patients and drugs at risk for QT prolongation: QT interval prolongation and arrhythmias have been a major cause for drug relabeling and withdrawals. However, only a small group of patients exposed to culprit drugs develops the ADR, and the fundamental determinants of this individual sensitivity remain unexplained.
In this project we are deriving cardiomyocytes from individual patients whose drug-response phenotypes we have established. We are comparing cells from those with drug-induced long QT syndrome (diLQTS) to those who have been drug-tolerant both at baseline and with exposure to drugs known to elicit this ADR, including HERG blockers as well as those inhibiting PI3-kinase, a new pathway to diLQTS we have recently delineated.