Current Research Projects
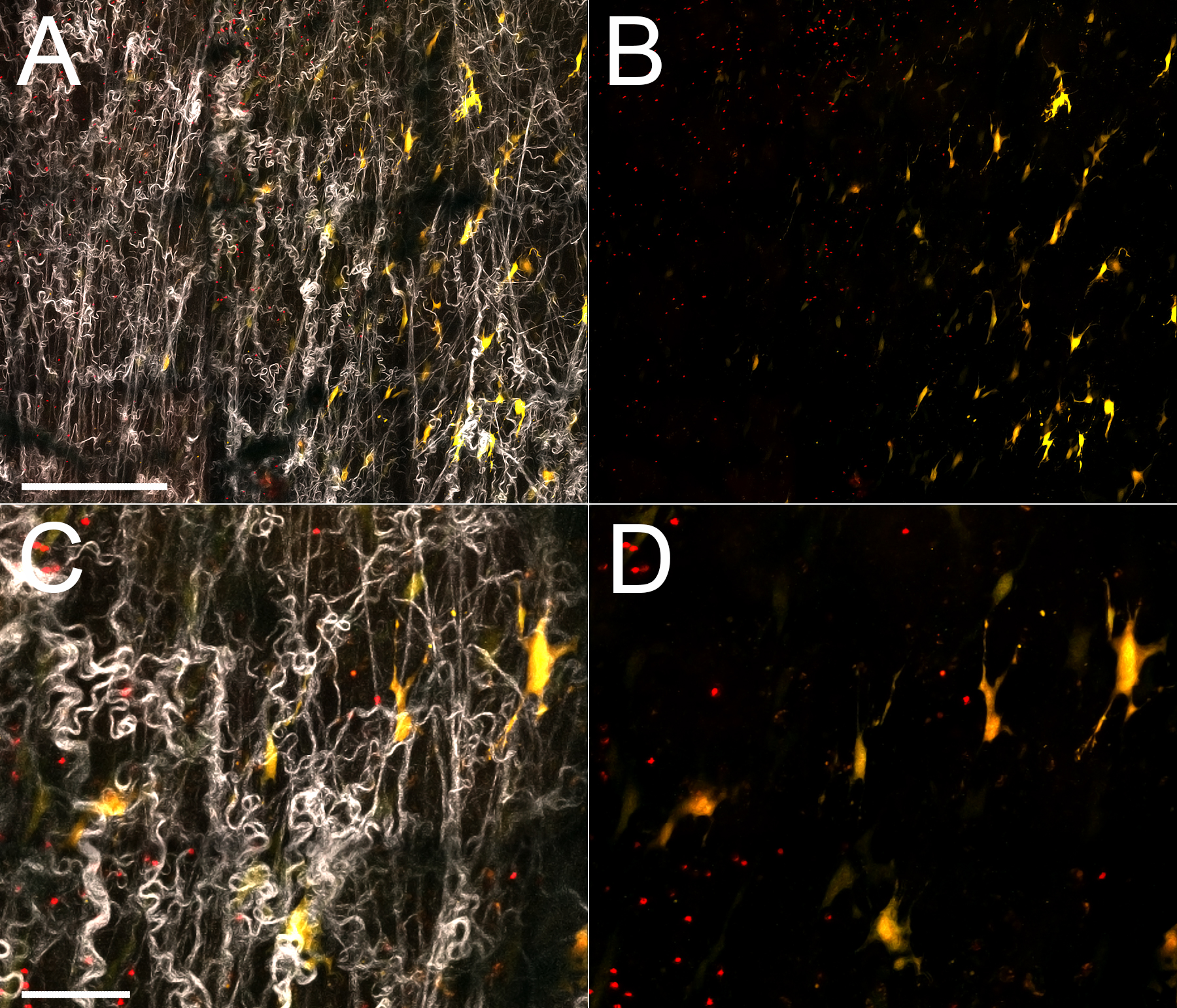
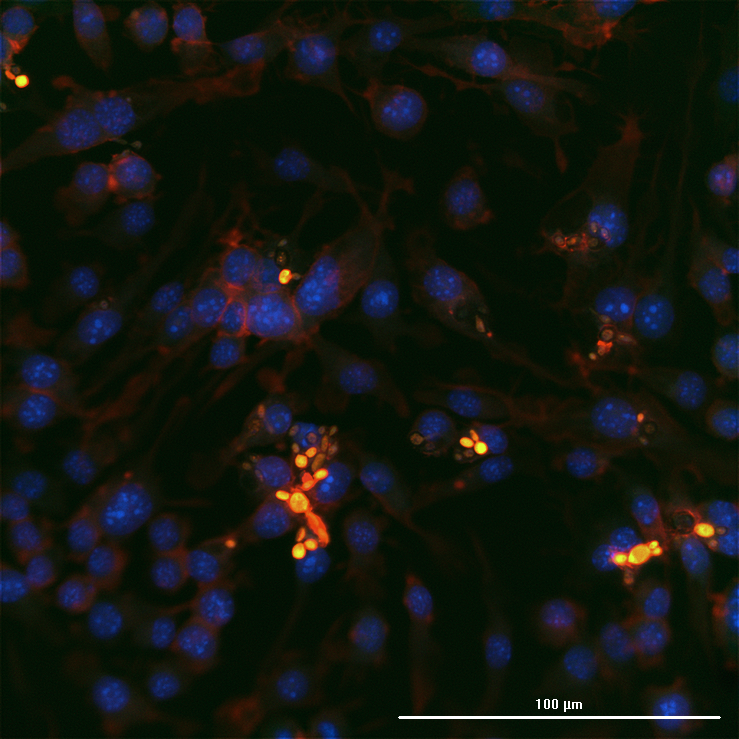
1 Skin host defense and inflammation
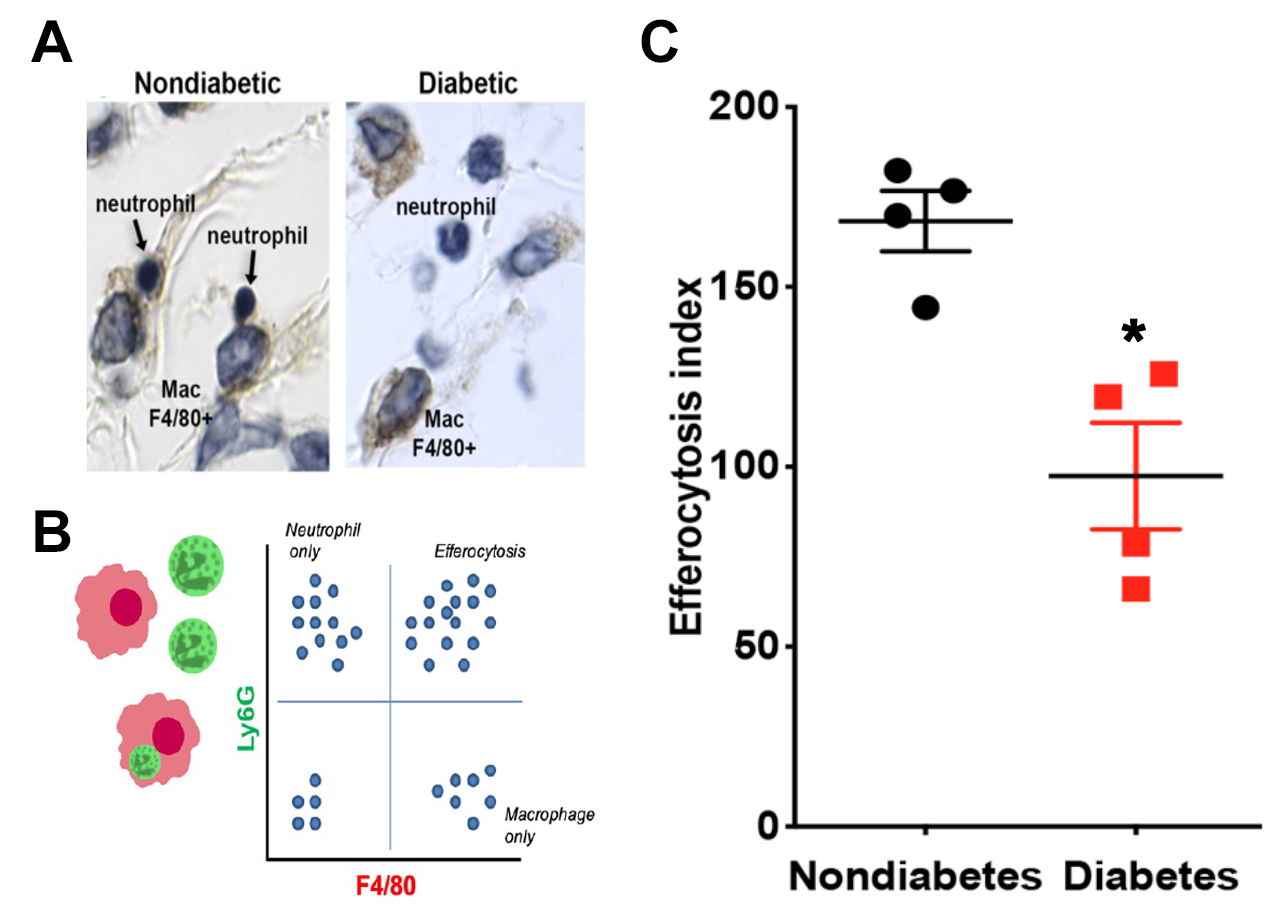
In vivo efferocytosis in the infected skin of diabetic and nondiabetic mice

2 Sepsis and organ damage in euglycemic and hyperglycemic conditions
People and mice with diabetes are more prone to sepsis. In human patients, the inflammatory response is exaggerated, leading to more severe organ damage and mortality. We have previously shown that increased mortality in septic diabetic mice is due to excessive lipid mediator leukotriene B4 (LTB4) production and the Myeloid differentiation primary response 88 (MyD88).
The Serezani lab is currently interested in investigating the organ-specific transcriptional and epigenetic landscape in phagocytes from infected diabetic and nondiabetic mice. We are also testing if the same programs are present in PBMC from people with sepsis and metabolic dysfunction.
We aim to understand how metabolic changes influence the abundance of metabolites required for epigenetic enzyme activation and the aberrant inflammatory response observed in septic and diabetic mice and people.