Research Studies
at the
Vanderbilt Psoriatic Arthritis and Spondyloarthritis Center
Psoriatic Arthritis Research Consortium (PARC)
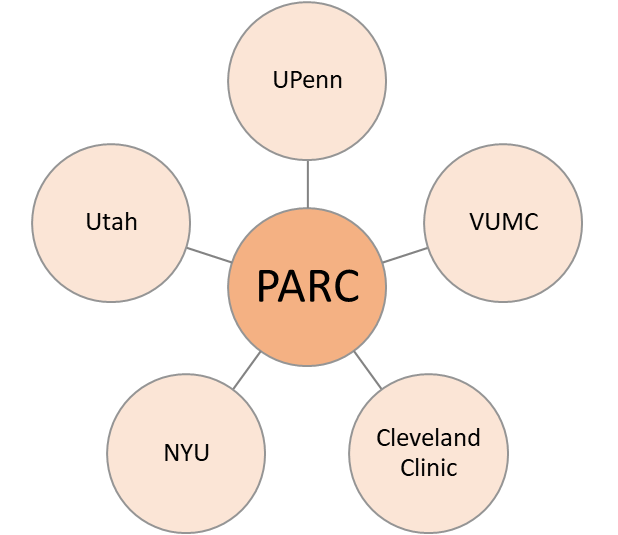
PARC is a longitudinal observational cohort that includes PsA-specialty clinics at five US institutions: the University of Pennsylvania, Cleveland Clinic, New York University, University of Utah, and Vanderbilt University.
The aims of this research study are to:
- Better understand the different subtypes (appearances) of psoriatic arthritis, psoriasis and related diseases
- Build a registry of data for future studies designed to identify the best therapies for people with each subtype of psoriatic arthritis and related diseases
- Determine how to best measure disease activity and quality of life for those with PsA.
NEtwork for Research and Development in Spondyloarthritis (NERDS)
Studying the various phenotypes of spondyloarthritis requires large numbers of patients so we have formed a collaboration with multiple spondyloarthritis clinics in the US.
This multi-center study aims to:
- Establish data resources for pragmatic research with spondyloarthritis
- Identify and validate appropriate outcome measures for pragmatic trials in spondyloarthritis
- Examine phenotypes, natural disease history, comorbidities, disease impact, therapies, and health care patterns with SpA and related diseases
Both PARC and NERDS are observational studies that doesn’t include any intervention. If a participant decides to enroll in the study, the doctor will ask questions about their health and do a physical exam. Information from these activities will be entered into a secured scientific database. Information that could identify the participant will not be included. Additionally, the participants could decide to provide blood (no more than 62 mL of blood) for further characterization of the disease.
Contact – Research Coordinator Sarah Wood, inflammatoryarthritis@vumc.org
Psoriasis and Psoriatic Arthritis Electronic Health Record (EHR) Cohorts
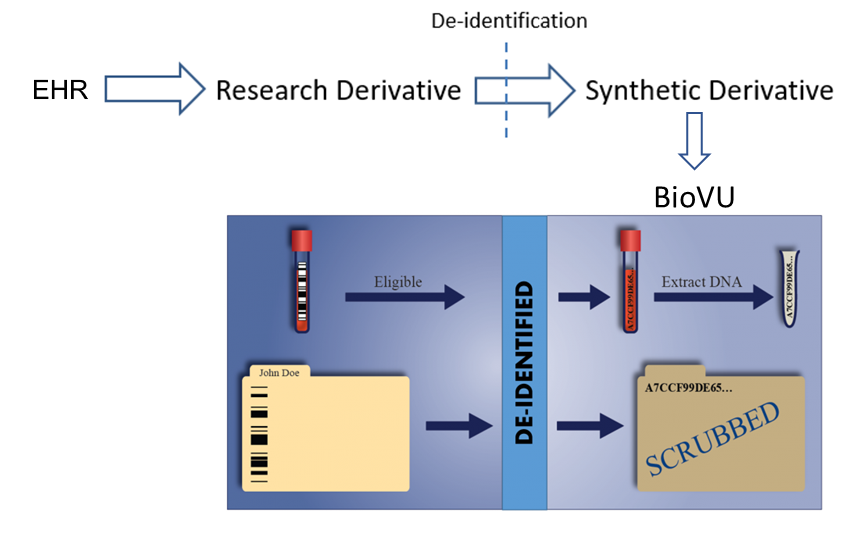
We have identified psoriasis and psoriatic arthritis patients in the de-identified version of Vanderbilt’s EHR called the Synthetic Derivative (SD, containing ~3.5 million subjects). The SD includes longitudinal clinical data over several decades (1993-2023) with all information available in the EHR: diagnostic and procedure codes, demographics, text from inpatient and outpatient notes (including both primary care and specialty), laboratory data, radiology reports, and medication orders.
The Synthetic Derivative can run text-based searches of the entire clinical record within seconds, improving the efficiency and accuracy of data extraction. These records are linked to a DNA biorepository called BioVU.
BioVU incorporates de-identified data from the EHR and genotype information to identify factors that affect disease susceptibility, disease progression, and drug response. Using the psoriasis and psoriatic arthritis cohorts, we are studying clinical and genetic factors associated with the development of psoriatic arthritis in patients with psoriasis.
We are also studying which combination of clinical and genetic factors are associated with better or worse treatment responses. Identifying these associations could help clinicians risk-stratify PsA patients to better predict the disease course and craft personalized management strategies.