Research
Our research program focuses on understanding how epithelium responds to injury and how normal injury response processes are subverted in the development of malignancy. The research activities span the basic to translational spectrum.
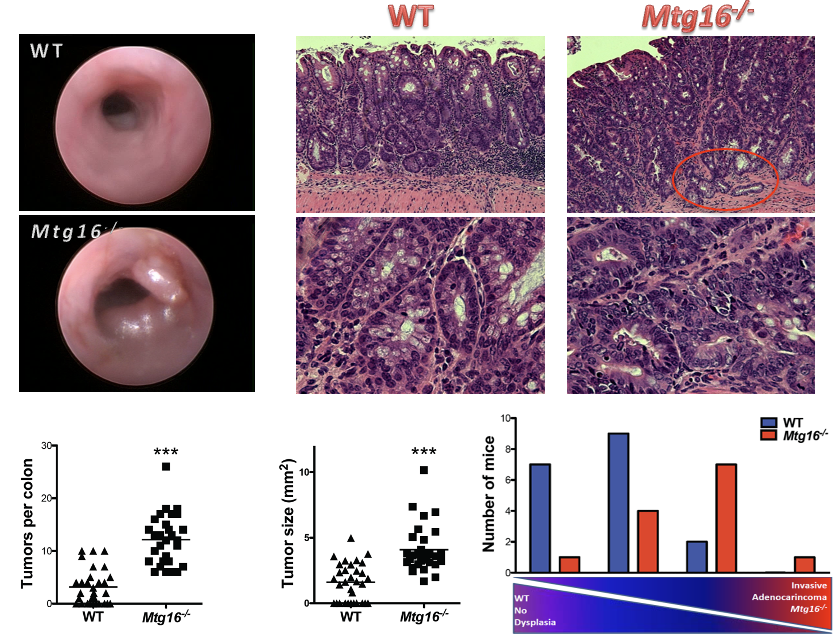
Epigenetic control of intestinal epithelial wound healing and repair programs and relationship to colorectal oncogenesis
We are studying the role of the Myeloid Translocation Gene (MTGs) family in intestinal biology with emphasis on gut development, stem cell function, wound healing and malignancy. MTGR1 (Myeloid Translocation Gene, Related-1), MTG8 and MTG16 are members of a gene family originally identified as targets of chromosomal translocation in acute myeloid leukemia (AML). MTG family members act as transcriptional repressors and interact with other corepressors mSin3, N-CoR/SMRT and histone deacetylases (HDAC1-3).
Chromosomal translocations often target master regulatory genes that affect growth, differentiation and apoptosis. Using unique mouse and 3D organoid models, we are characterizing MTGs as modifiers of WNT-dependent tumorigenesis in the gut and determining how they regulate stem cell programs.
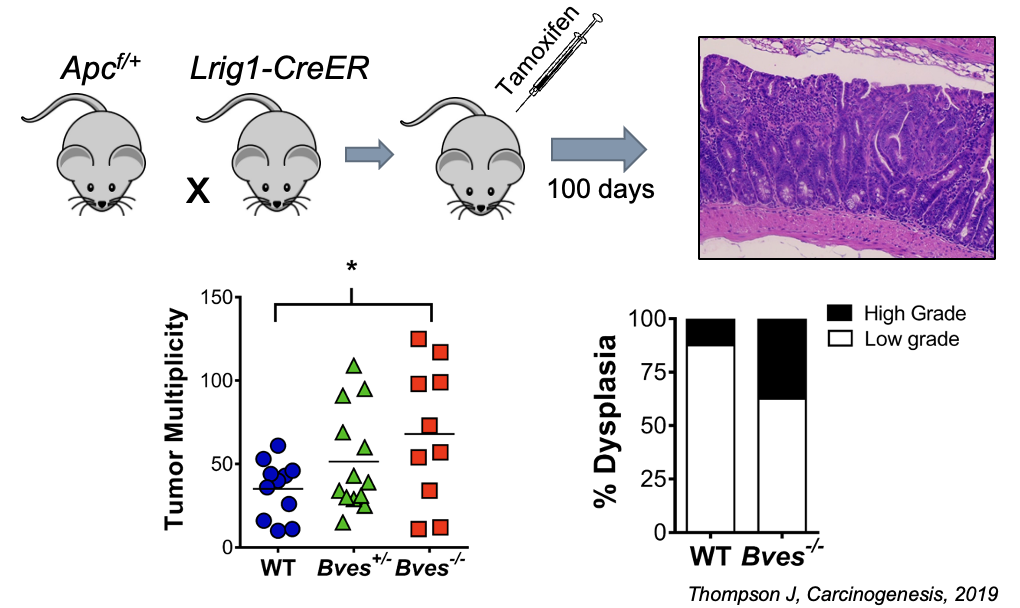
Junctional signaling in mucosal wound healing responses and inflammatory carcinogenesis
We determined that a tight junction-associated protein called BVES was suppressed by promoter hypermethylation in colon cancer and that it regulates epithelial to mesenchymal transition (EMT), influencing metastasis. We are currently cataloging the BVES protein interactome as a means to understand how BVES transduces "Out to In" signaling. In addition, using Bves-null mice and organoids, we are dissecting its role in intestinal wound healing using chemical, infectious, and mechanical colonic injury modeling and its role in colitis-associated cancer.
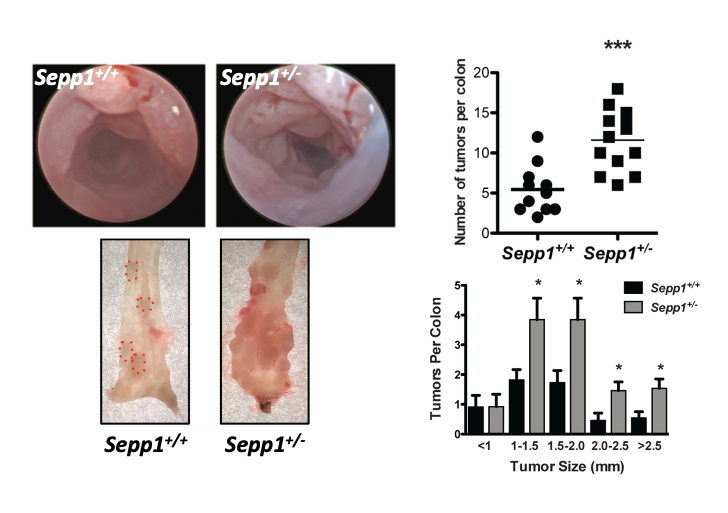
Oxidative injury defenses in inflammatory bowel disease (IBD) and colitis associated carcinoma (CAC)
Little is known about the role of selenium and selenoproteins in inflammatory bowel disease (IBD), a condition of intermittent severe oxidative stress. We have shown that selenium deficiency results in increased mucosal injury and progression to colitis-associated dysplasia (CAD). Likewise, germline Sepp1 and Gpx3 mutant mice had increased tumor burden with major effects on proliferation, apoptosis and DNA damage.
We hypothesize that intestinal Sepp1 alters the inflammatory microenvironment via clearance of reactive oxygen species thus affecting epithelial stem cell and differentiation programs. We are determining which stem cell pathways are modified by selenoproteins in influencing tumorigenesis.