Boothby Lab Research
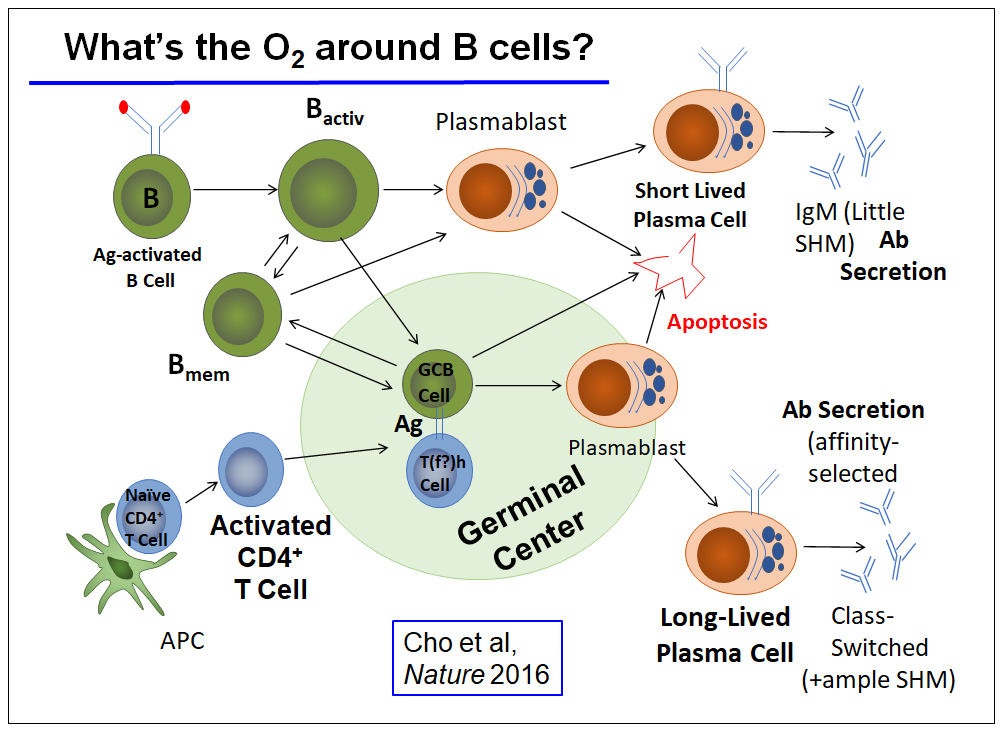
What's the O2 around B Cells?
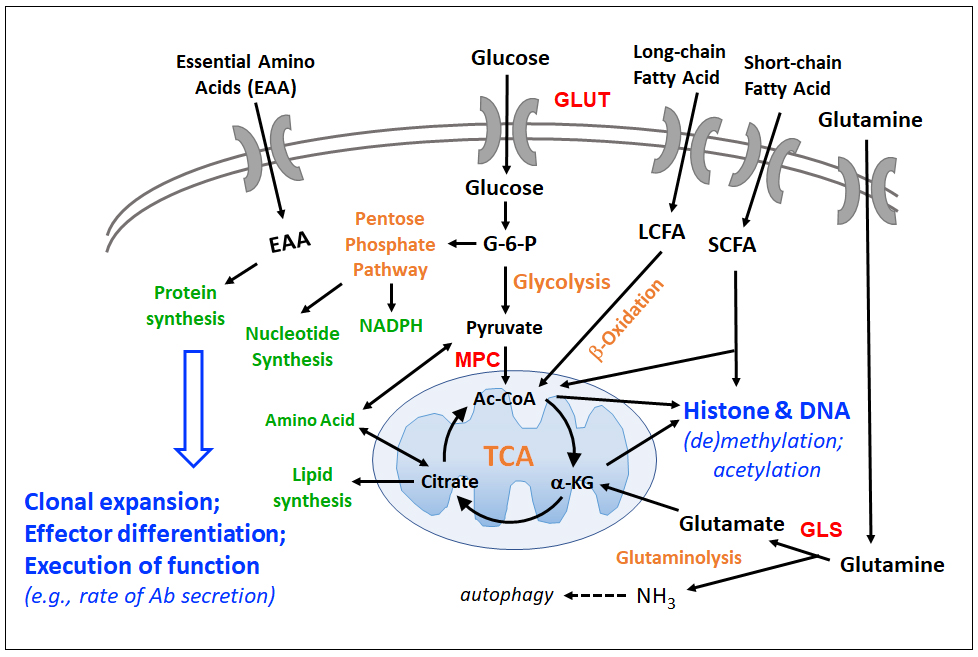
We first explored how the main parameter that could be estimated on a practical scale in a micro-anatomical milieu – oxygen – is distributed in lymphoid follicles during normal immune physiology.
The striking findings, replicated independently and in parallel by others, sharpened a recognition that natural variation in oxygen availability and hypoxia-inducible transcription factors such as HIF-1 may modulate physiological immunity. In addition, these studies include the mTOR complexes (mTORC1, 2), AMP-activated protein kinase (AMPK), and hypoxia-inducible transcription factors (HIF) as regulated by oxygen and metabolite sensitive dioxygenase enzymes.
The past decade of work has engaged heavily in analyzing how these pathways and the nutrient micro-environment affect germinal centers, overall qualities of antibody responses, and each limb of humoral memory.
Regulation prove essential to advancing therapeutic goals in humans
In a seminal paper in which the Boothby Lab established that IL-4 receptor signals via a rapamycin-sensitive pathway (Wang DZ et al, Journal of Biological Chemistry 1995, 1997), the lab developed toward work dissecting the contributions of specific nutrient sensor systems in B and T cell responses.
Our work on IL-4 signaling and a member of the PARP protein family, led me a dozen years ago to start to explore how effects on intermediary metabolism and flux rates can be the targets of signal transducers that then influence survival, proliferation, and differentiation in B lineage cells.
In parallel, collaborations with cancer biologists - especially work in the area of carcinomas and solid tumors – and interactions with a colleague (O. McGuinness), who is rigorous and deeply knowledgeable about metabolism through his T2D research, stimulated analyses of the interplay between supplies of extracellular nutrients, signaling, and function.