Biobanking and Sample Processing
Experienced and accredited
The Cardiology Core Laboratory for Translational and Clinical Research offers:
- Well-established infrastructure and Standard Operating Procedures (SOPs) for specimen collection, processing, inventory, and storage.
- Support for investigator-initiated biobanking projects, as well as multi-center clinical trial biobanking
- Assurance to follow the biobanking guidelines established by the National Heart, Lung, and Blood Institute (NHLBI) and the International Society for Biological and Environmental Repositories (ISBER)
- Biorepository accreditation from the College of American Pathology (CAP)
Multi-Center Clinical Trial Biobanking
Central biobanking for clinical trials including lab manual set up, assembling and shipping specimen collection kits to sites, sample receiving, biobanking, and database set up.


Sample Processing
- Biological sample processing and storage – blood, urine and tissue
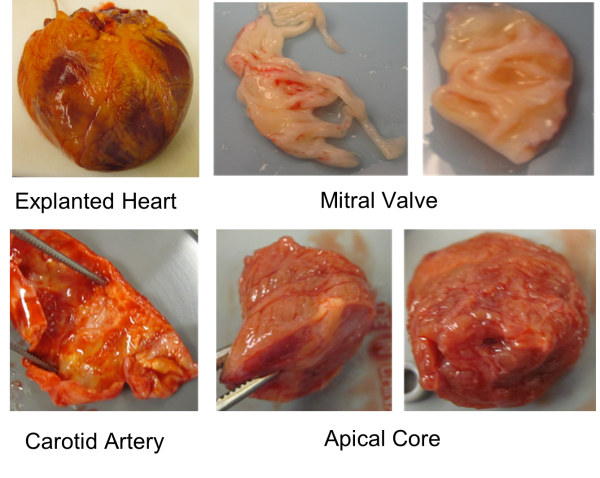
- Human heart tissue sample processing and storage – flash frozen, OCT sections, formalin-fixed paraffin-embedded eections (picture of tissue samples that are now in Biobanking)
- DNA & RNA Extractions from saliva, tissue and blood
- Clinical sample shipping & handling