Featured Publications
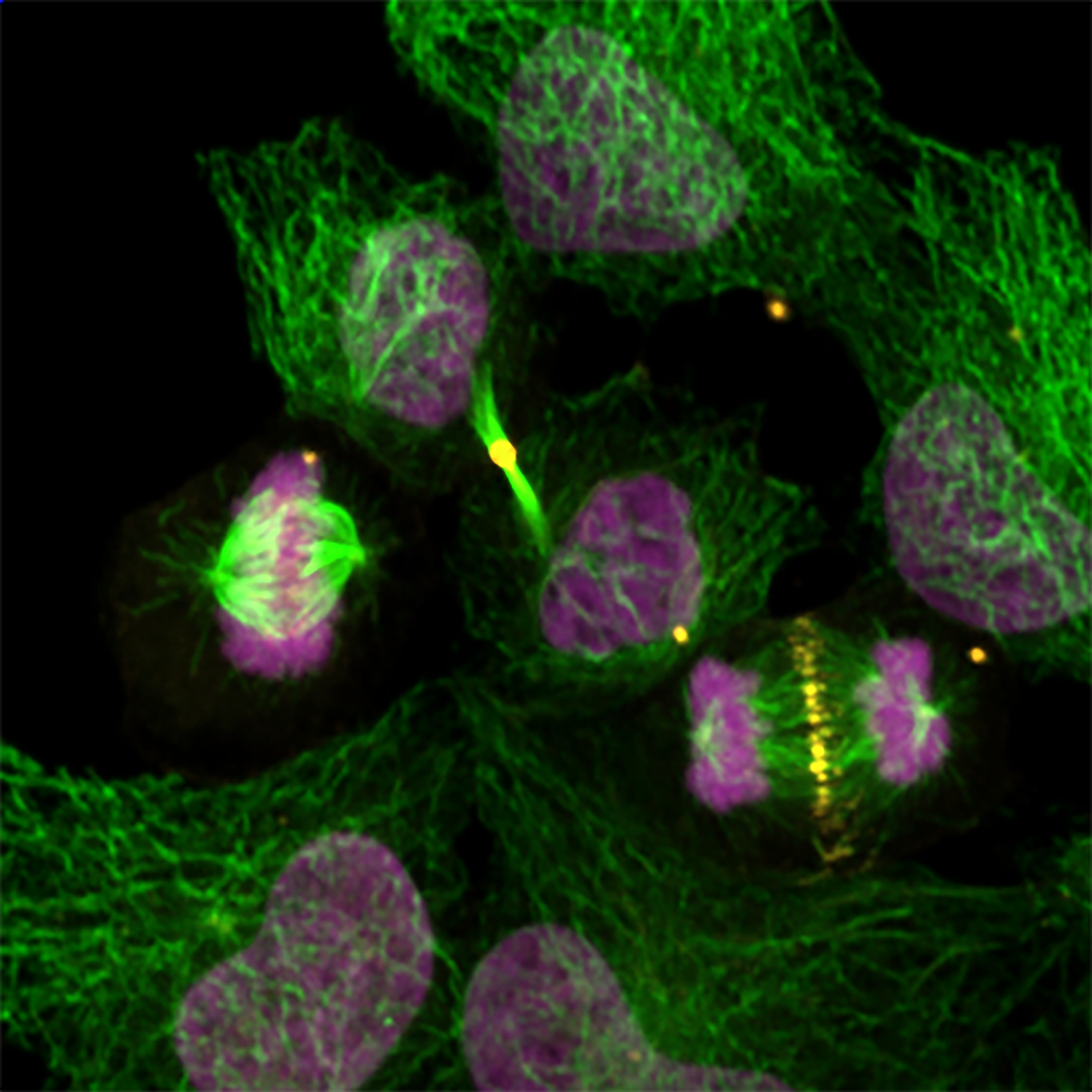
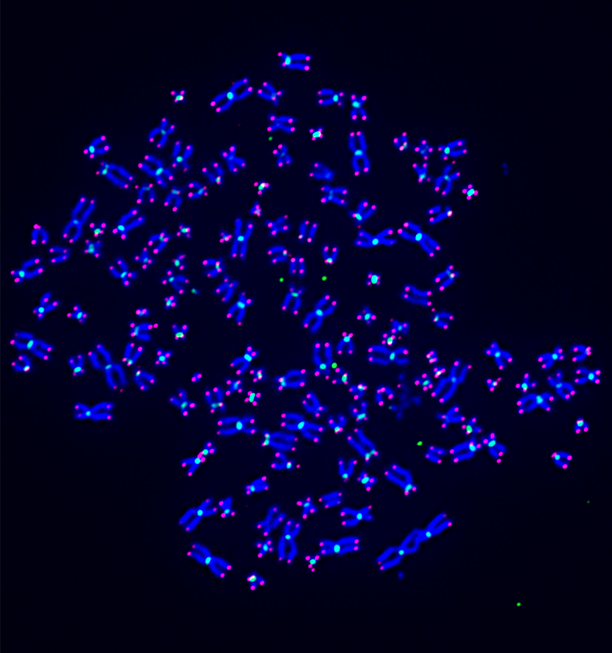
Research in the Frank Mason Lab identified that loss of the tumor suppressor SETD2 or its catalytic activity led to the generation of an abnormal chromosome structure called isochromosomes, or chromosomes with mirror-imaged symmetry. These rare, palindromic chromosomes occur in cancer and developmental disorders, but mechanisms for the origins in mammalian cells are entirely unknown.
We found that, during DNA replication, SETD2 prevents the formation of isochromosomes, and other chromosomal aberrations, by recruiting RAD51 to promote homologous recombination, and in the absence of SETD2, the more error prone RAD52 is utilized to generate these chromosomal rearrangements that promote copy number variation.
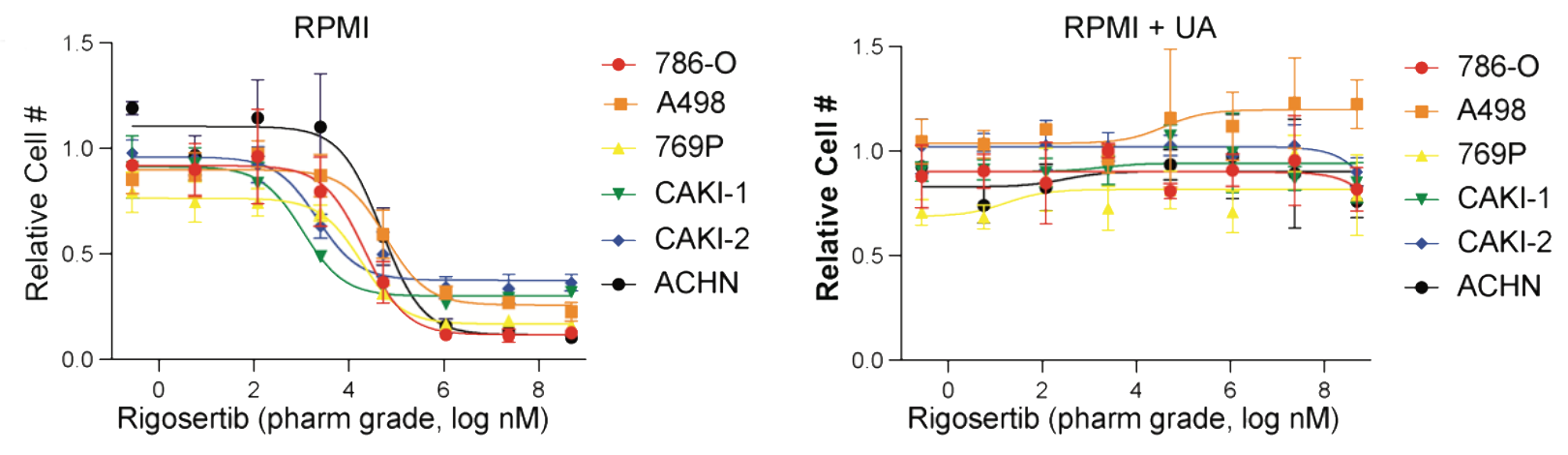
Drug screening in human physiologic medium identifies uric acid as an inhibitor of rigosertib efficacy
JCI Insight 2024
In collaboration with the Coloff Lab at University of Illinois, Chicago, the Mason Lab identified that the metabolic waste product uric acid prevents the ability of rigosertib to depolymerize microtubules and promote cell death. Rigosertib has long held promise as a cancer therapeutic, as it has remarkable potency in in vitro and in vivo cancer models but has had very little success in patients.
By screening FDA-approved compounds in traditional versus human plasma-like media (HPLM), which contains nutrients at physiological concentrations, we identified that Rigosertib does not promote cell death in HPLM due to the presence of uric acid, the purine metabolism end product. Uric acid is not normally present in conventional medium nor in mice, explaining the variance with tumor models and patient response.
The Mason Lab’s previous work, in collaboration with Cheryl Walker (Baylor) and Kimryn Rathmell (director of the National Cancer Institute, formerly Chair of Medicine at VUMC), identified that SETD2 can methylate a-tubulin, a component of the microtubule cytoskeleton during mitosis.
Here, we demonstrate that SETD2 recognizes the C-terminal tail of a-tubulin, and upon binding, prefers to methylate soluble tubulin rather than polymerized microtubules. We additionally identified mutations in the C-terminal SRI domain of SETD2 that differentiate between a-tubulin and RNA Polymerase II binding. This work was done in collaboration with Michael Cianfrocco and Kristen Verhey (University of Michigan).