1. CMV is important in aging; what keeps Cytomegalovirus (CMV) -specific T cells inflated?
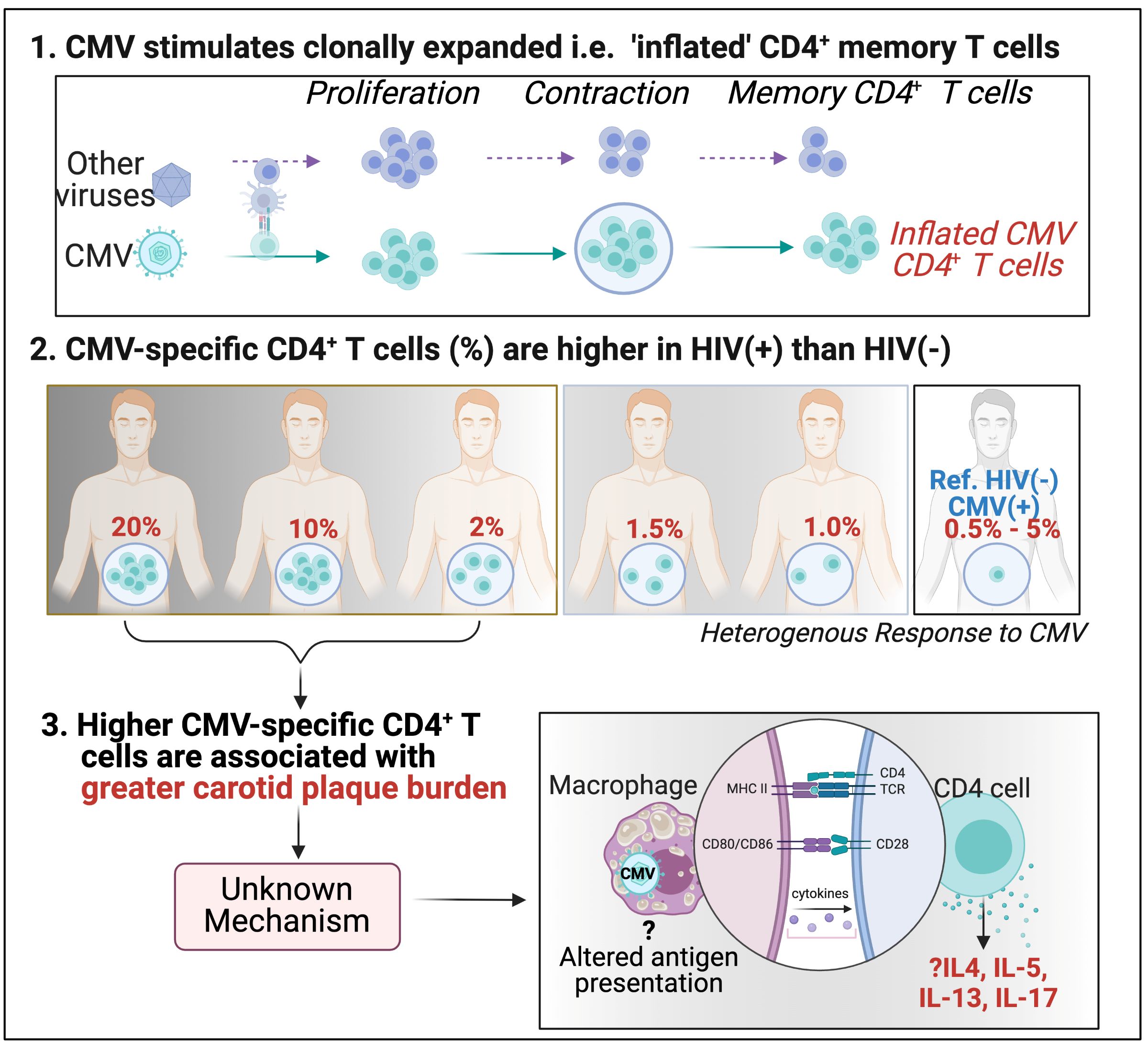
CMV stimulates clonally expanded i.e. 'inflated' CD4+ memory T cells.
CMV-specific CD4+ T cells % are higher in HIV(+) than in HIV(-).
Higher CMV-specific CD4+ T cells are associated with greater carotid plaque burden.
2. Characterize antigens and immune cells within coronary arteries and adipose tissue
Goal: To characterize the antigens that promote inflammation in coronary arteries and the immune cell phenotype in atherosclerotic plaque and perivascular adipose tissue, bearing in mind the role of human genetics and other risk factors, including environmental exposures.

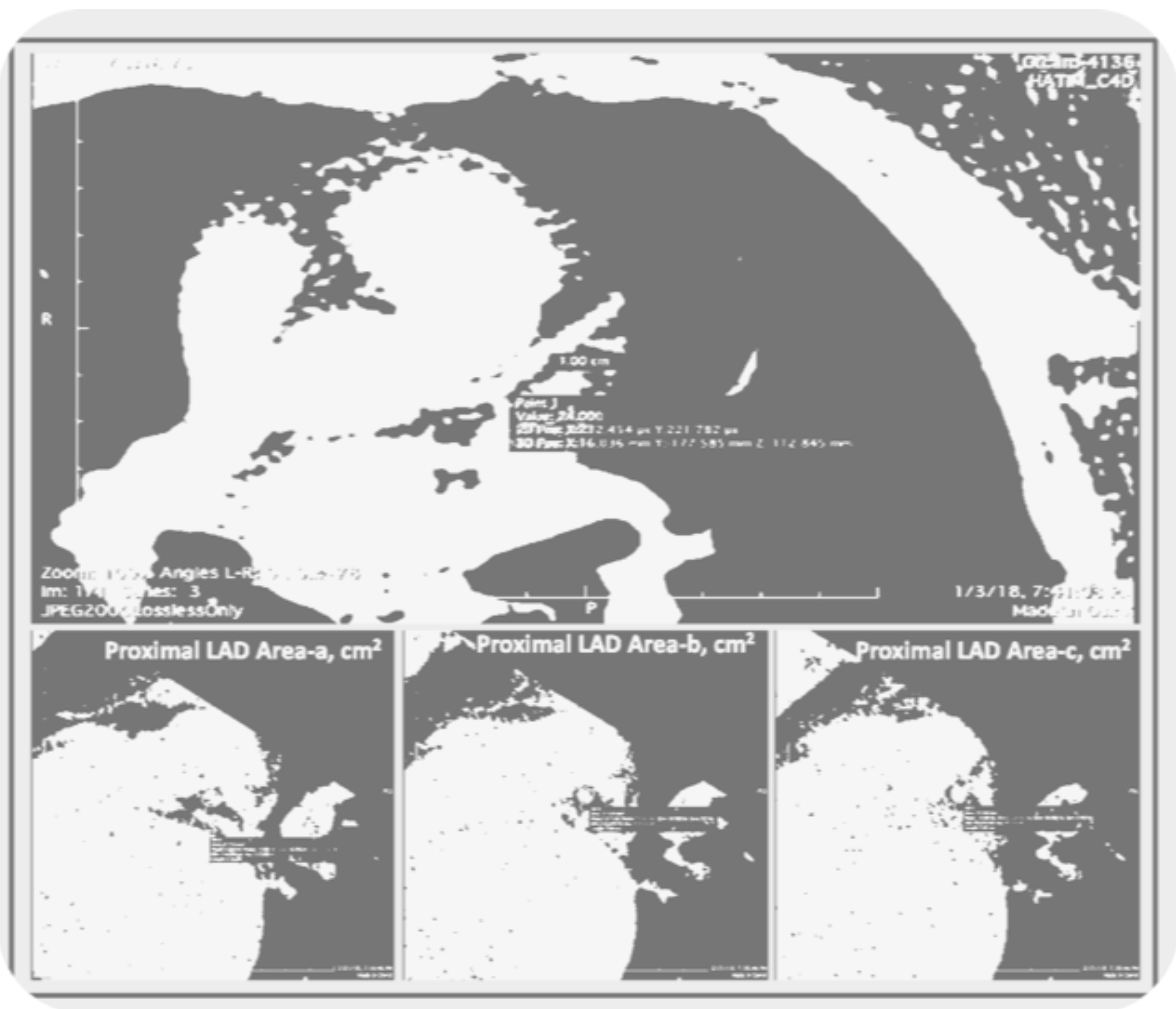
3. Mean Coronary Cross-Sectional Area as a Measure of Arterial Remodeling
Mean coronary cross-sectional area (CorCSA) measured through non-contrast CT imaging can be used to assess arterial remodeling and potential atherosclerosis.
Our goal is to optimize existing tools and find novel ways of studying CVD and defining clinical endpoints in our cohort studies.
Read the article Mean Coronary Cross-Sectional Area as a Measure of Arterial Remodeling Using Noncontrast CT Imaging in Persons With HIV
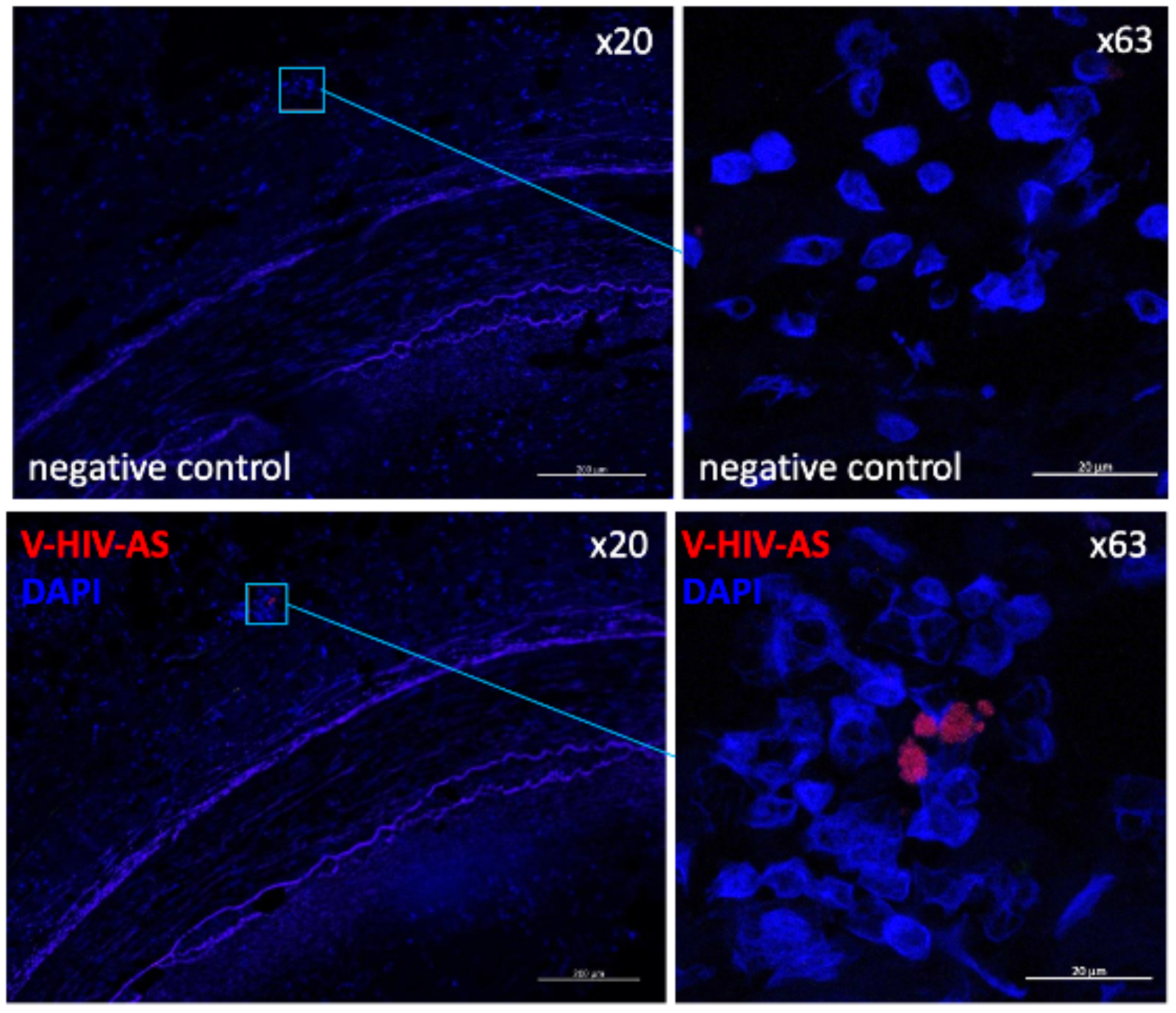
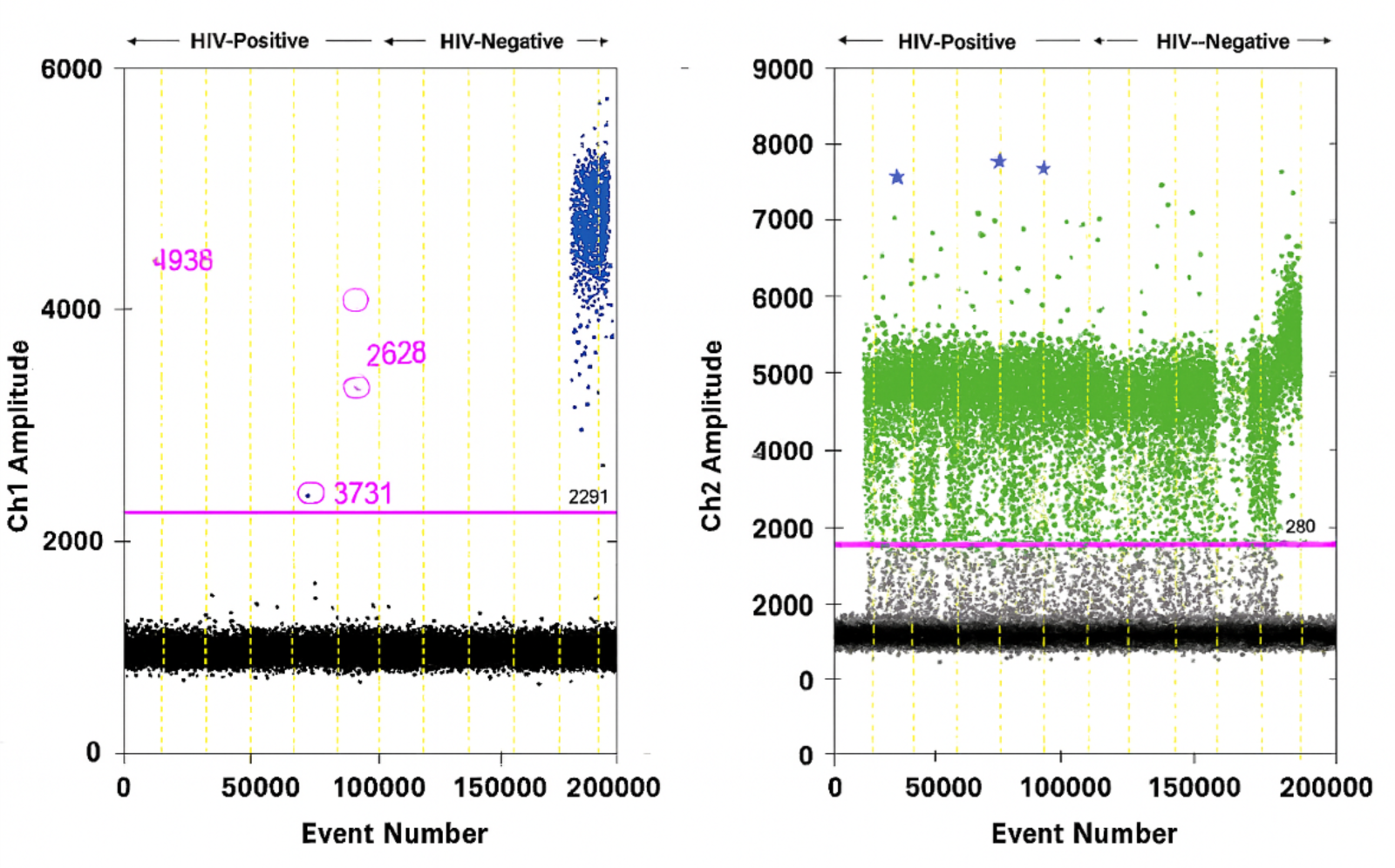
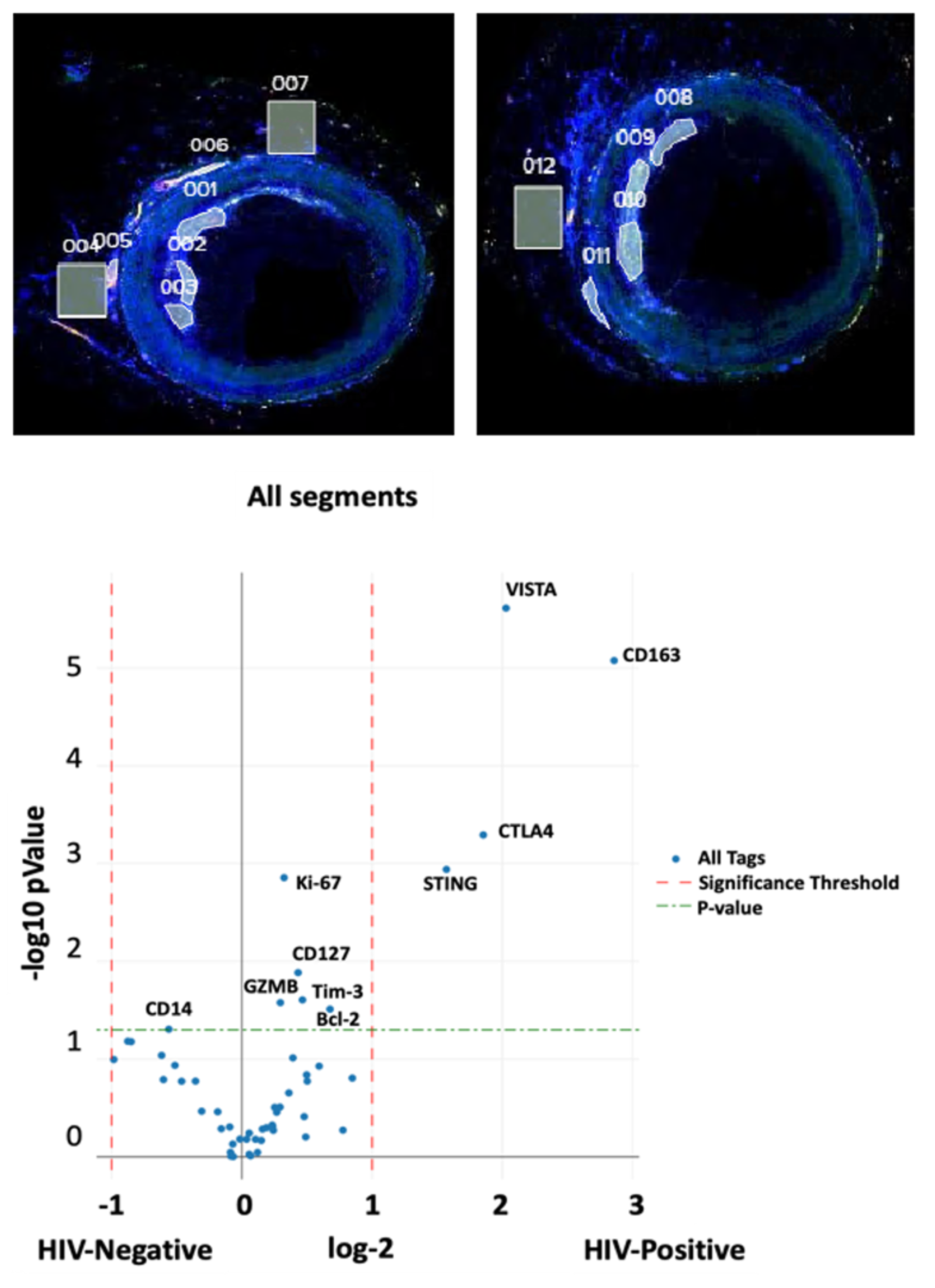
4. What is the role of chronic viral reservoirs in the recruitment of immune cells into atheroma?
Several human studies have detected CMV in arterial plaque, while others have failed to do so. A recent publication showed that monocytes latently infected by CMV had lower expression of MHC class II and its chaperone CD74. This was accompanied by an anergic-like state with a slightly weaker immune response. HIV-infected smooth muscle cells in atherosclerotic plaques have also been reported.
We propose that monocytes/macrophages/dendritic cells within coronary plaques are infected with HIV and CMV, which alters their function. The goals of this research are to:
- Identify virus-infected immune cells in coronary plaques
- Simultaneously evaluate the RNA transcriptome of monocytes/macrophages, T cells, and smooth muscle cells using digital spatial profiling
- Analyze the TCR repertoire of T cells in coronary plaques.
Read more:
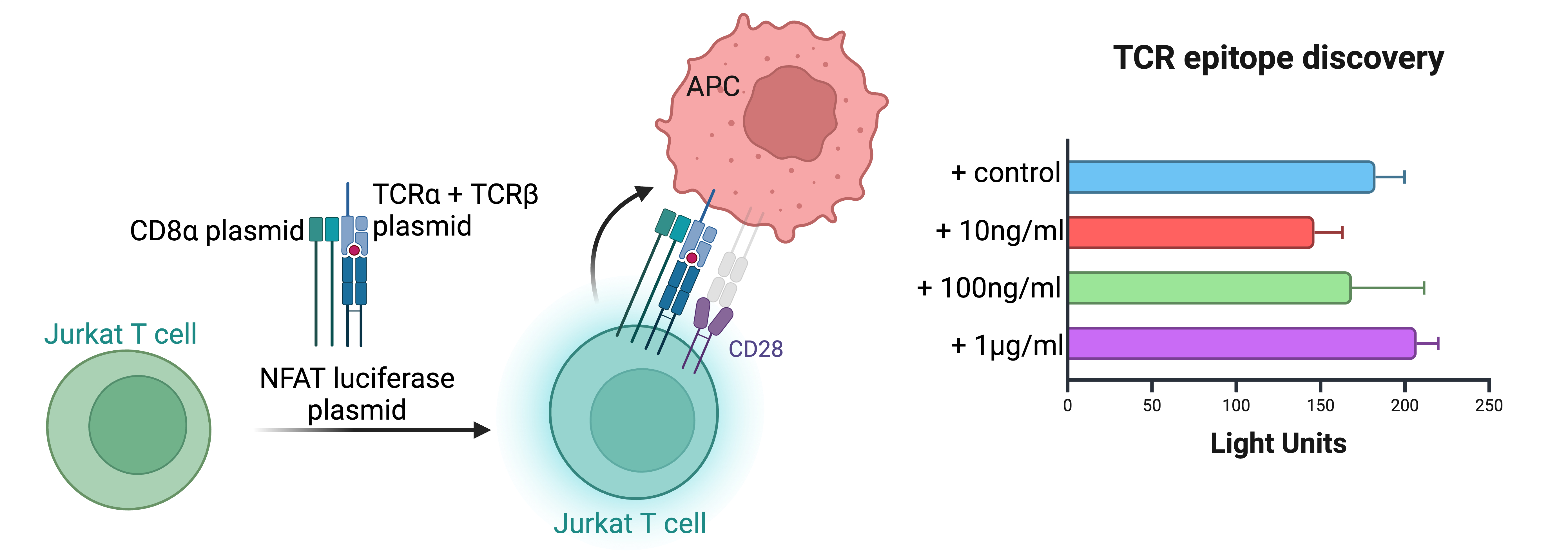
5. Antigen specificity of plaque infiltrating CGC+ CD4+ T cells
Atherosclerosis is an immune-mediated disease with evidence of T cells in fatty streaks and early and late fibroatheroma. Atherosclerotic plaques from persons with symptomatic CAD have distinct CD4+ T cell subsets such as Th1, thought to be pro-atherogenic and significant for plaque rupture. The roles of Th2 and Th17 cells, among other CD4 subsets, are unclear.
CD28- CD4+ T cells, which overlap with CMV-specific CGC+ CD4+ T cells, are present in atherosclerotic plaques. Our preliminary data showed that CD28- CD4+ T cells were associated with incident CVD in PWH.
By obtaining coronary artery and coronary plaque samples from study participants, we aim to isolate CD4 and CD8 T cells from tissue, and, using chimeric Jurkat T cell lines, express synthetic dominant TCR a/b pairs to establish the antigen specificity of the T cells (virus and modified self-antigens)
6. Study the effect of Cytomegalovirus (CMV) on endothelial cell dysfunction
Human CMV can infect human aorta endothelial cells in culture. We think that this is happening in vivo and alters the function of the cells through multiple mechanisms that we are currently studying.

7. Study the effect of cytokines expressed by CGC+ CD4+ T cells on endothelial cell inflammation
We think that inflammatory cytokines drive subclinical atherosclerosis in persons with HIV. Cells that express chemokine receptors that home cells to endothelium are likely to influence endothelial health more strongly due to their local effects.
8. Studying the role of different immune cell subsets in cardiometabolic disease incidence
This a research project aims at investigating the role of different subsets of immune cells from human participants in the development of cardiometabolic disease using mouse models.
Leveraging the NSG mouse model and human immune cells, we are investigating the causal effect of different immune subsets in the pathogenesis of cardiometabolic disease.
This project is led by Victoria Stephens, PhD.
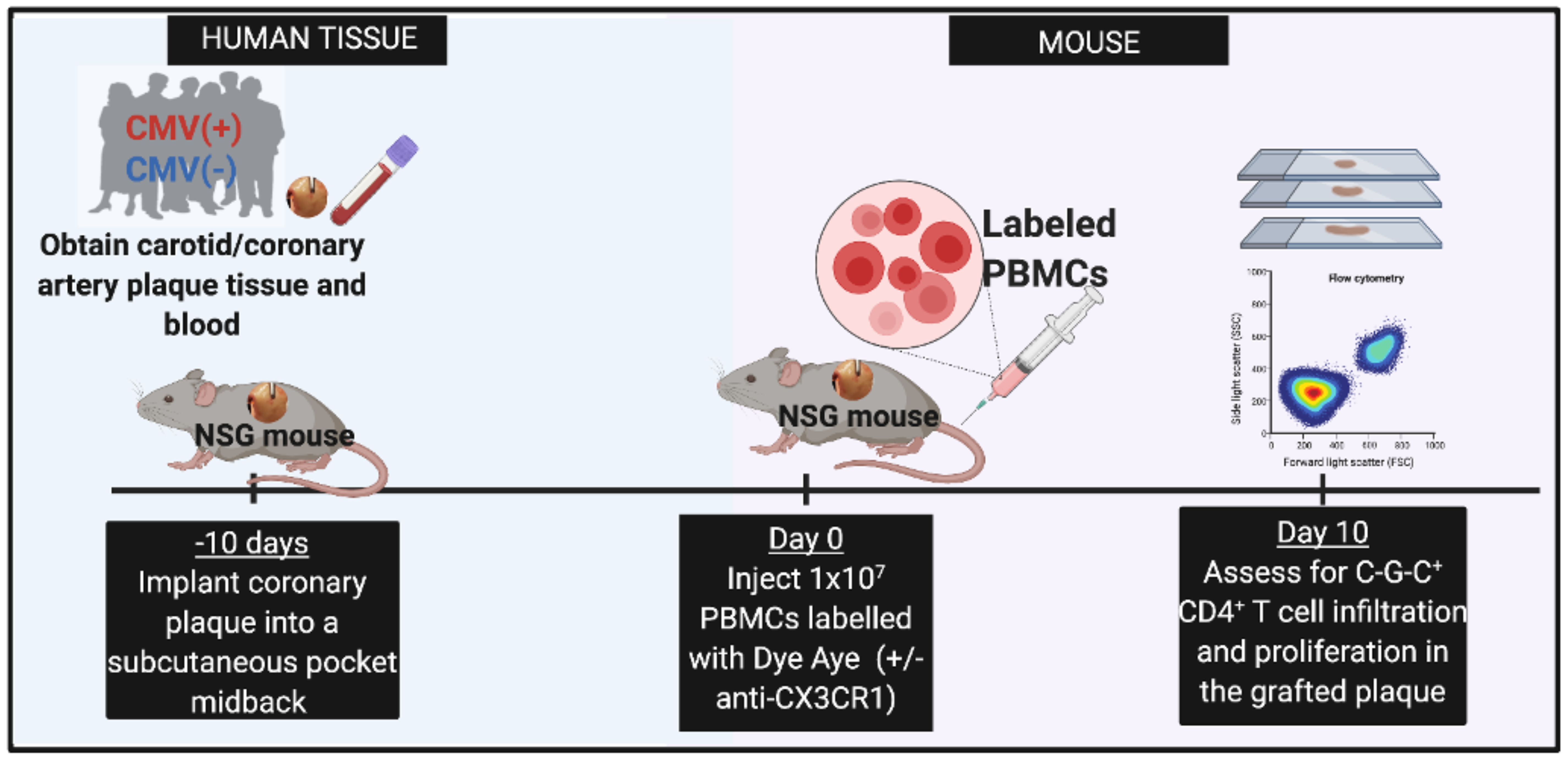
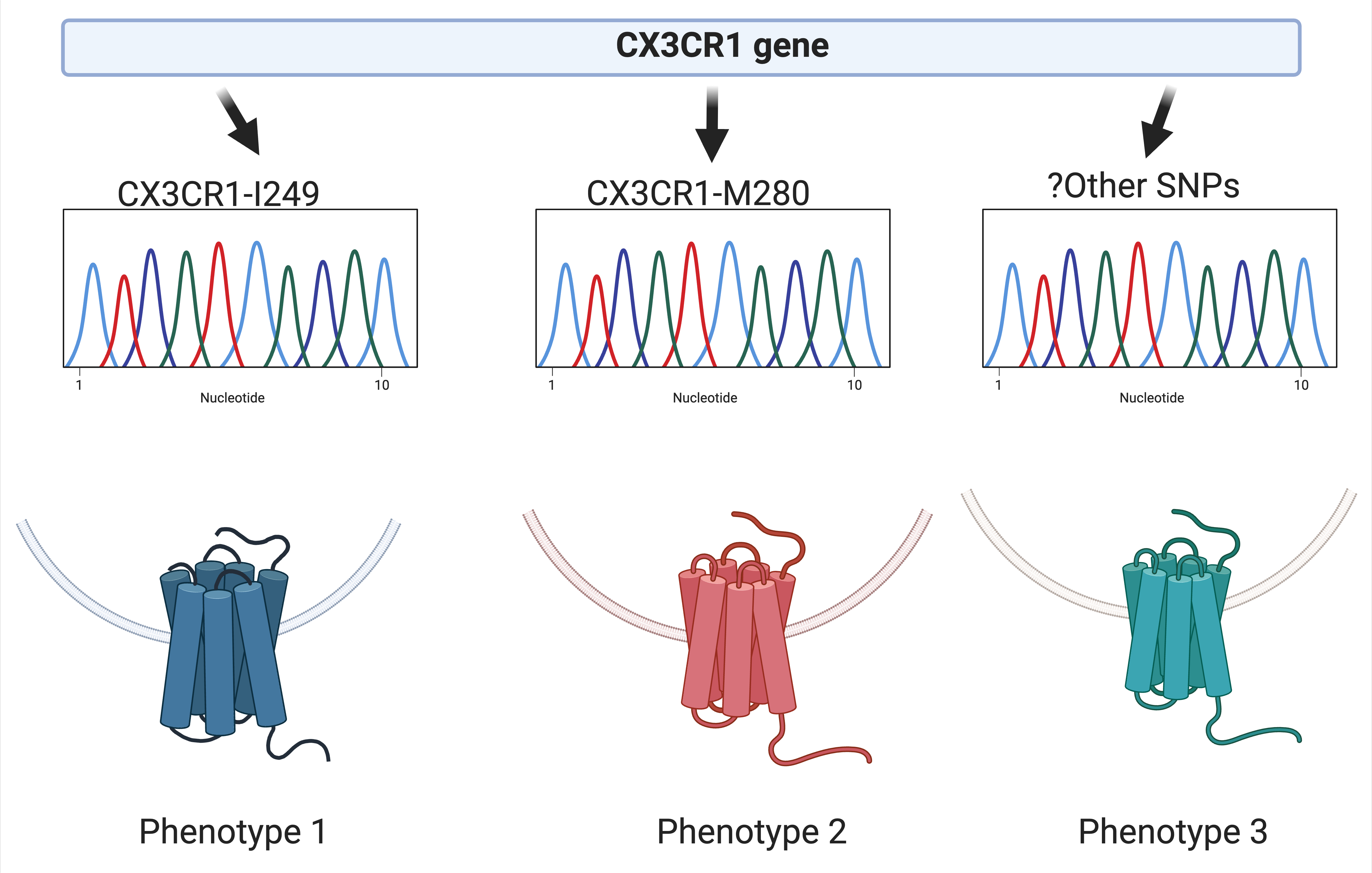
9. CX3CR1 and cardiovascular disease in persons with HIV
This study aims to investigate whether inhibiting CX3CR1 decreases the recruitment of immune cells to atherosclerotic plaque or adipose tissue.
This project is led by Ronald McMillan, PhD.
10. B cell subsets and atherosclerosis in persons with HIV (PWH)
Previous mouse and human studies have defined B cells with atheroprotective and atherogenic properties among the different cell types implicated in cardiovascular disease. For example, B1 cells express natural antibodies against low-density lipoproteins that can be atheroprotective yet decrease with age. Depleting atherogenic B2 cells in murine studies attenuated diet-induced atherogenesis, suggesting that B cells could be a therapeutic target to reduce the progression of atherosclerotic lesions.
There have been no studies that have defined the role of B cells in atherosclerosis in PWH. HIV affects over 38 million people globally, and deaths from CVD continue to rise in this group, highlighting the need for innovative strategies to reduce morbidity and mortality.
This project is led by Laventa M. Obare, MD.
11. Studying the role of immune complexes in cardiometabolic disease and cancer among PLWH
Traditional flow cytometry methods have previously excluded cells that were not singlets from analysis, due to concern that they represented artifacts of immune cell processing. However, a study by Burrel showed that in fact many of these complexes were actually T cells complexed with monocytes and were increased after vaccination or during acute infections. We showed that in people with chronic HIV infection, these complexes are increased in people with pre-diabetes and diabetes.
Furthermore, we have captured these cells through time-lapse imaging and sown that some are transient, while others remain in complex over time. Moreover, more copies of HIV were detected in these complexes compared to matched CD4 T cells. To understand the antigenic or inflammatory promoters of complexes, and how they change over time with cardiometabolic disease or cancer, we will be studying these cells in longitudinal cohorts from the FAR Network of Integrated Clinical Systems (CNICS).