Publications
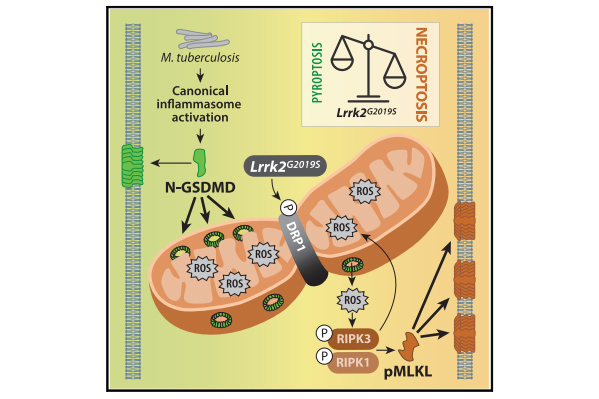
Mitochondrial ROS promotes susceptibility to infection via gasdermin D-mediated necroptosis
Weindel et al., Cell
Although mutations in mitochondrial-associated genes are linked to inflammation and susceptibility to infection, their mechanistic contributions to immune outcomes remain ill-defined. We discovered that the disease-associated gain-of-function allele Lrrk2G2019S (leucine-rich repeat kinase 2) perturbs mitochondrial homeostasis and reprograms cell death pathways in macrophages.
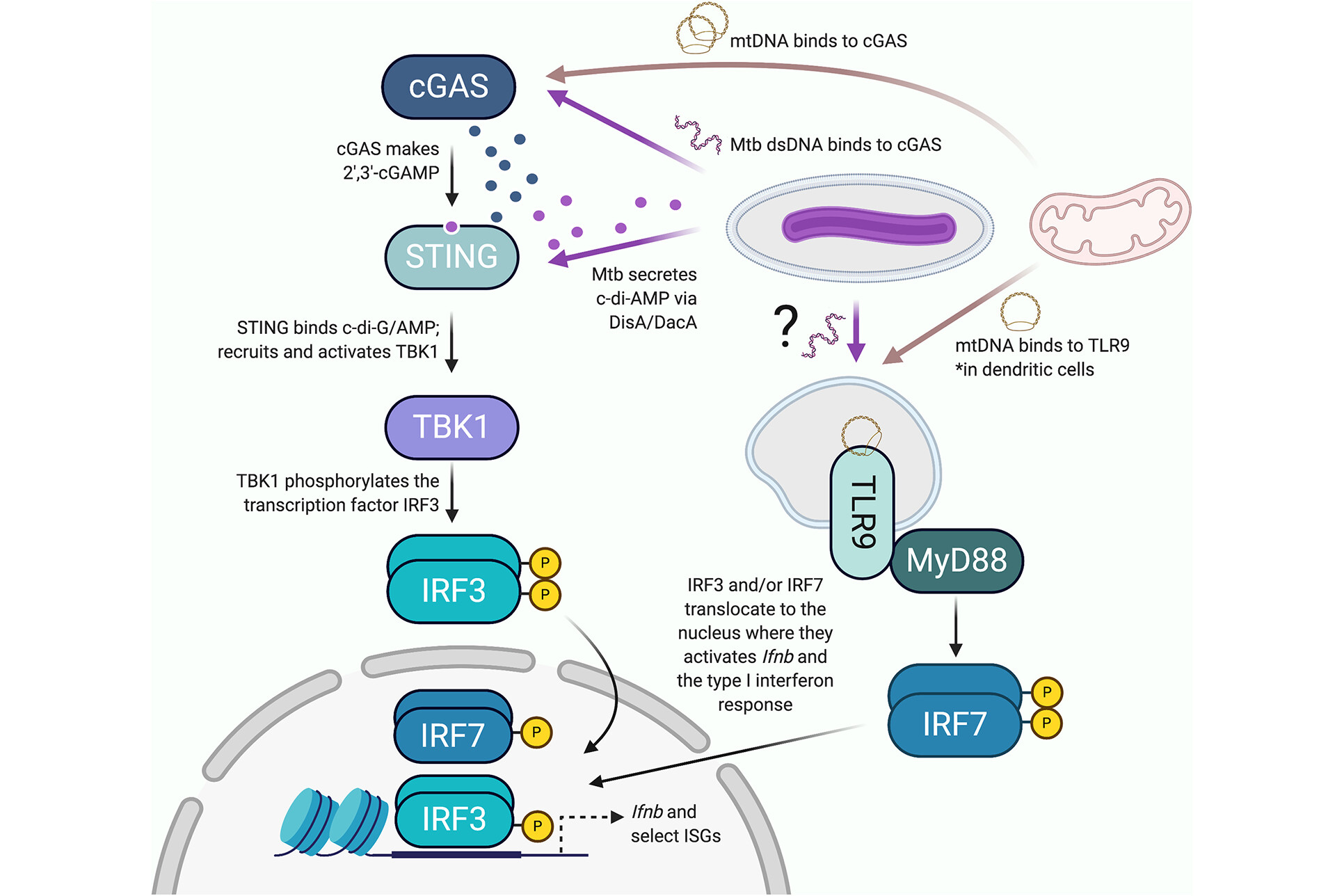
Mitochondria: Powering the Innate Immune Response to Mycobacterium tuberculosis infection
Patrick and Watson, Infection and Immunity
Within the last decade, we have learned that damaged mitochondria activate many of the same innate immune pathways that evolved to sense and respond to intracellular pathogens. These shared responses include cytosolic nucleic acid sensing and type I interferon (IFN) expression, inflammasome activation that leads to pyroptosis, and selective autophagy (called mitophagy when mitochondria are the cargo).
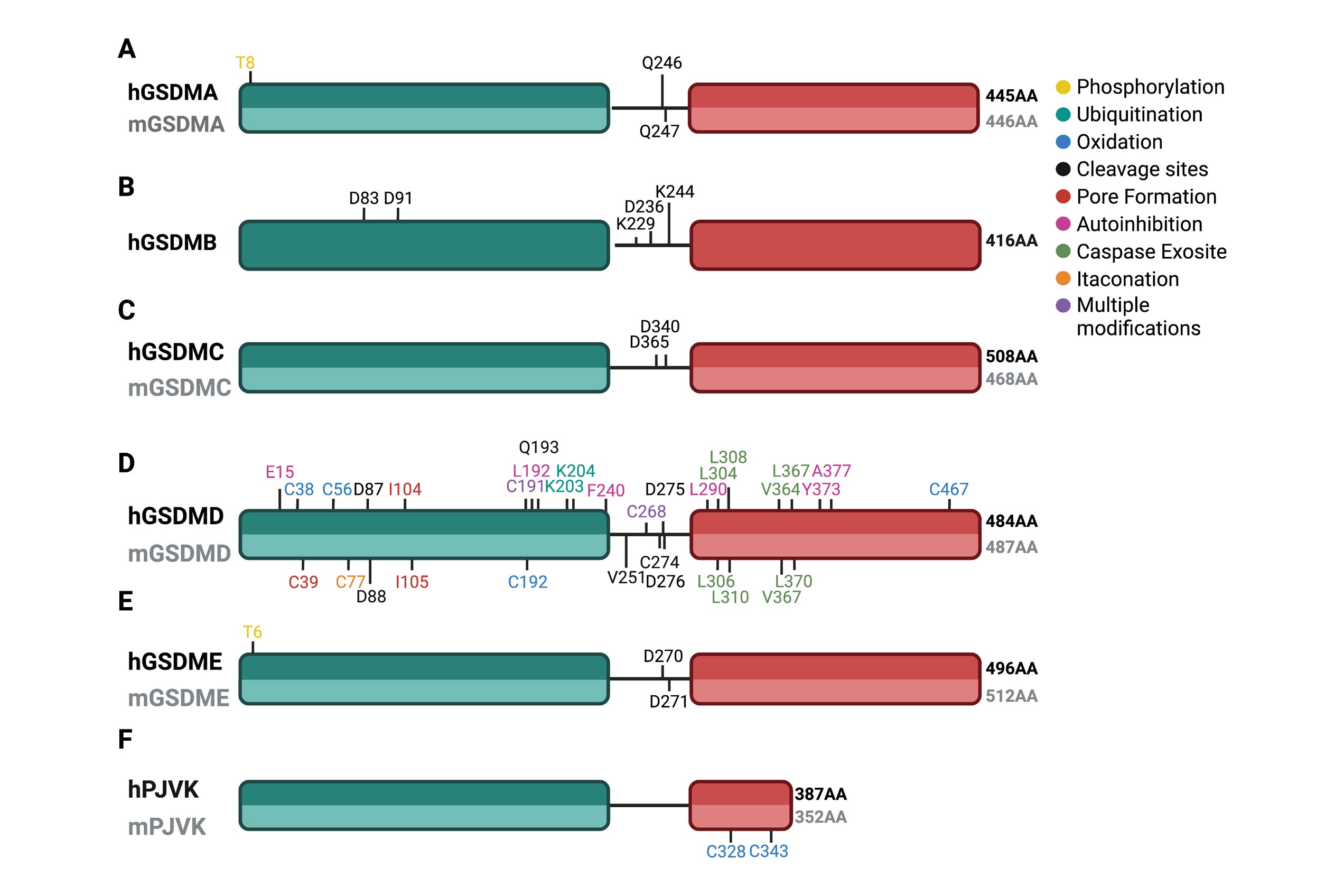
Gasdermins gone wild: new roles for GSDMs in regulating cellular homeostasis
Weindel et al. Trends in Cell Biology
Since their discovery, members of the gasdermin (GSDM) family of proteins have been firmly established as executors of pyroptosis, with the N-terminal fragment of most GSDMs capable of forming pores in the plasma membrane. More recent findings suggest that some GSDMs can drive additional cell death pathways, such as apoptosis and necroptosis, through mechanisms independent of plasma membrane perforation.
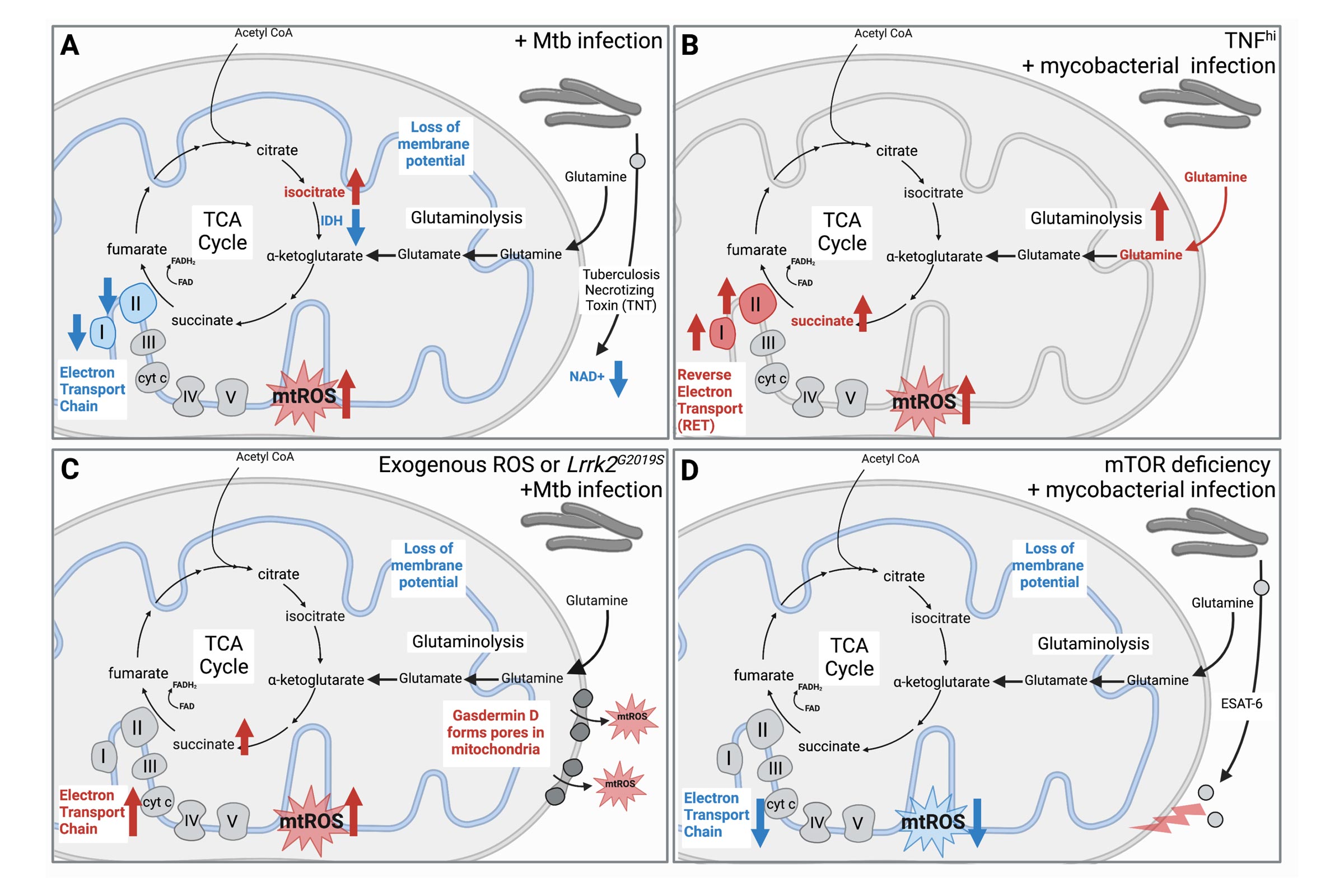
Mitochondrial reactive oxygen species: double agents in Mycobacterium tuberculosis infection
Ellzey et al., Current Opinion in Immunology
In addition to housing the major energy-producing pathways in cells, mitochondria are active players in innate immune responses. One critical way mitochondria fulfill this role is by releasing damage-associated molecular patterns (mtDAMPs) that are recognized by innate sensors to activate pathways including, but not limited to, cytokine expression, selective autophagy, and cell death.
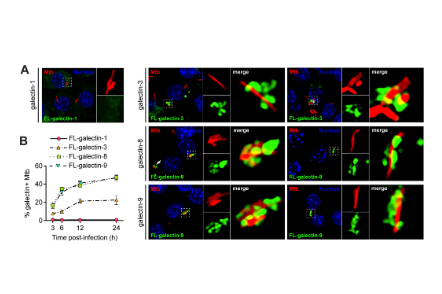
Galectin-8 senses phagosomal damage and recruits selective autophagy adapter Tax1BP1 to control Mycobacterium tuberculosis infection in macrophages
Bell et al., mBio
Mycobacterium tuberculosis (Mtb) causes one of the deadliest infectious diseases worldwide. Upon infection, Mtb is phagocytosed by macrophages and uses its virulence-associated ESX-1 secretion system to modulate the host cell.
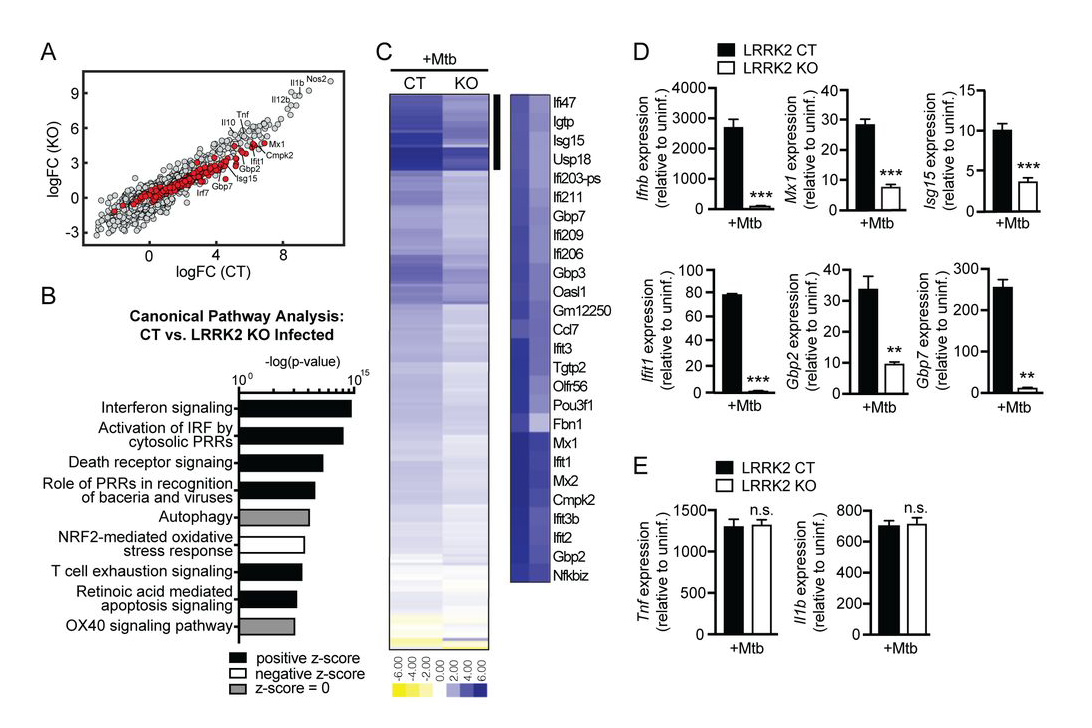
LRRK2 maintains mitochondrial homeostasis and regulates innate immune responses to Mycobacterium tuberculosis
Weindel, Bell et al., Elife
Despite many connections between mutations in leucine-rich repeat kinase 2 (LRRK2) and susceptibility to mycobacterial infection, we know little about its function outside of the brain, where it is studied in the context of Parkinson’s Disease (PD). Here, we report that LRRK2 controls peripheral macrophages and brain-resident glial cells’ ability to respond to and express inflammatory molecules.
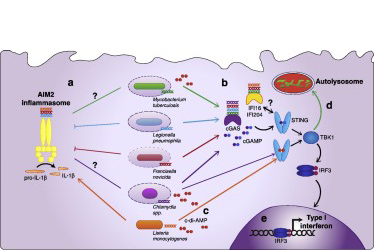
For Better or Worse: Cytosolic DNA Sensing during Intracellular Bacterial Infection Induces Potent Innate Immune Responses
Patrick, Bell et al., Journal of Molecular Biology
Many intracellular bacterial pathogens previously thought to remain sealed within a vacuole can be recognized by cytosolic innate immune sensors. While a wide array of cytosolic nucleic acid sensors have been characterized in the context of viral infection, we are only now beginning to examine how these same molecules function in the context of bacterial infection.
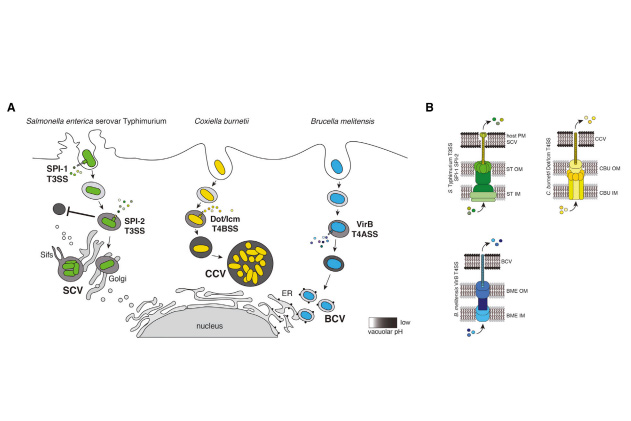
Quantitative yeast genetic interaction profiling of bacterial effector proteins uncovers a role for the human retromer in Salmonella infection
Patrick et al., Cell Systems
Despite playing a crucial role in virulence, the molecular targets of most bacterial effector proteins remain completely unknown. Using budding yeast as a heterologous host, we generated genetic interaction profiles for 36 bacterial effector proteins and validated target pathways/complexes predicted by our yeast screen with experiments in human cells.
TRIM14 is a Key Regulator of the Type I IFN response during Mycobacterium tuberculosis infection
Hoffpauir et al., Journal of Immunology
Tripartite motif-containing proteins (TRIMs) play a variety of recently described roles in innate immunity. While many TRIMs regulate type I interferon (IFN) expression following cytosolic nucleic acid sensing of viruses, their contribution to innate immune signaling and gene expression during bacterial infection remains largely unknown.