My lab is interested in understanding the mechanisms by which normal and malignant cells regulate programmed cell death. Multicellular organisms have devised a tightly regulated, genetically programmed mechanism of cell suicide to maintain homeostasis and to prevent propagation of genetically damaged cells. The discovery of the BCL-2 family of genes uncovered the underlying genetic mechanism of this regulation, as well as a class of oncogenes that governs cell death rather than cell proliferation.
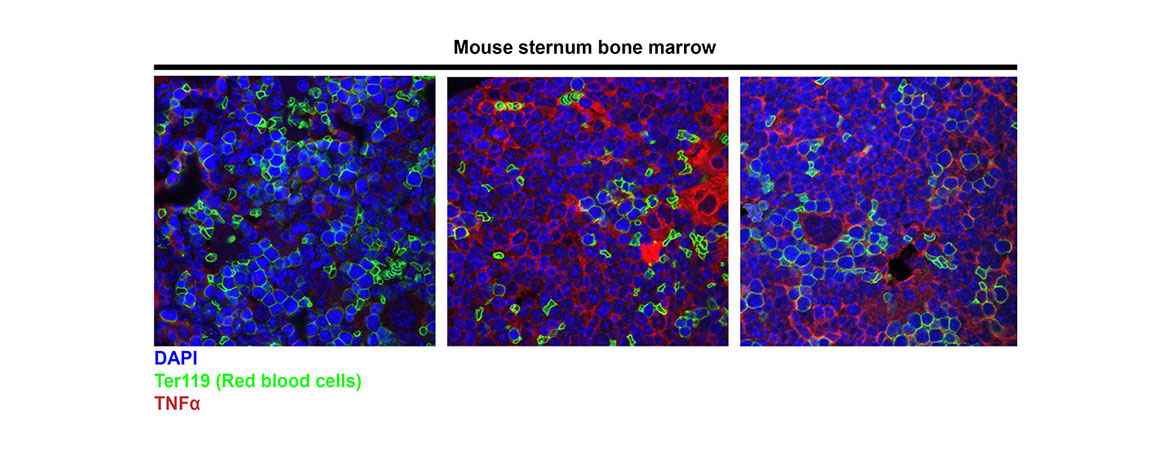
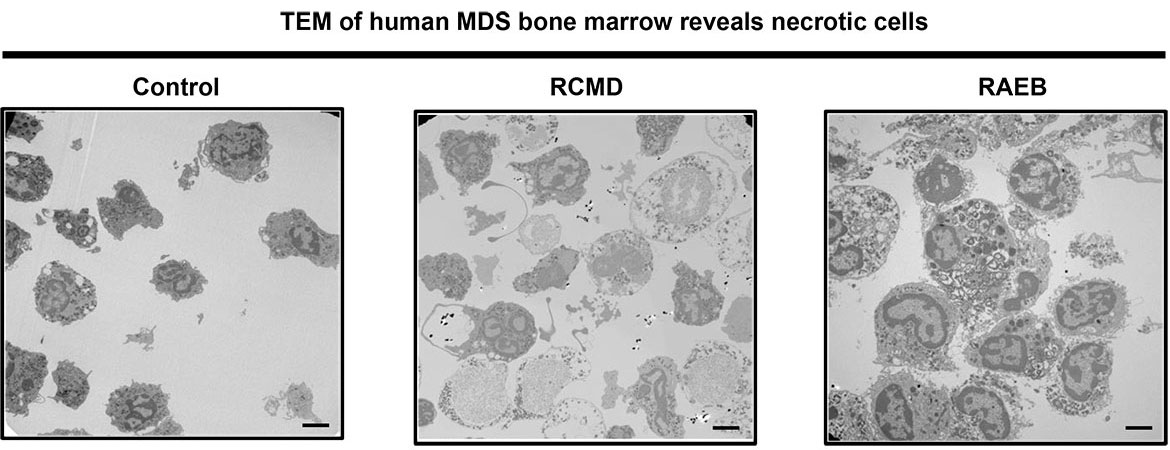
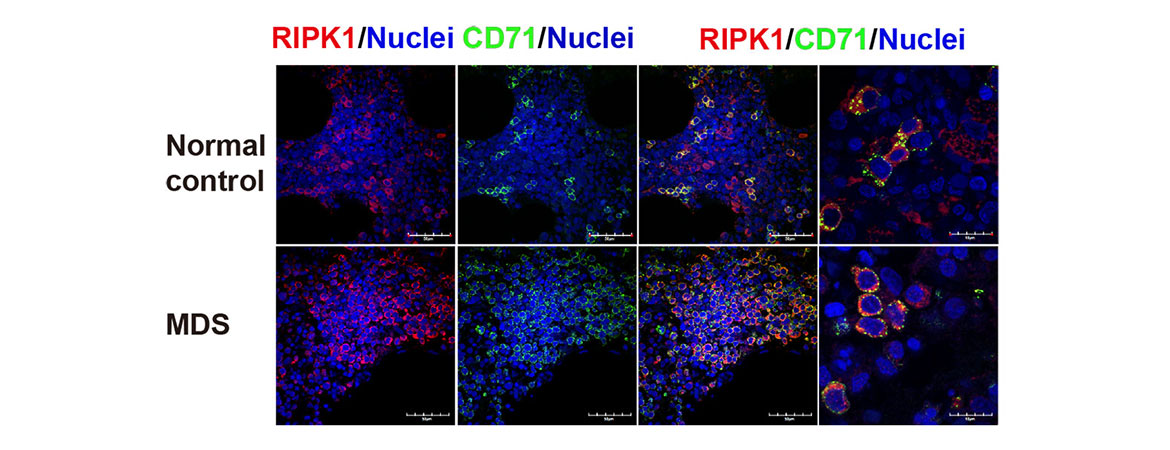
There are two major pathways that regulation programmed cell death: apoptosis and programmed necrosis. Simply, apoptotic cells implode in a relatively immune silent manner. Necrotic cells explode, releasing cellular contents and inciting an immune response- beneficial in settings of infection, but detrimental in settings of chronic damage, where the inflammation elicited by necrotic cell death amplifies cellular damage. Current studies focus on how programmed cell death regulates homeostasis in the hematopoietic (blood) system. We have found that unrestrained programmed necrosis leads to bone marrow failure in mice that closely resembles the human disease Myelodysplastic syndrome (MDS), and find increased necrosis in human MDS bone marrow.
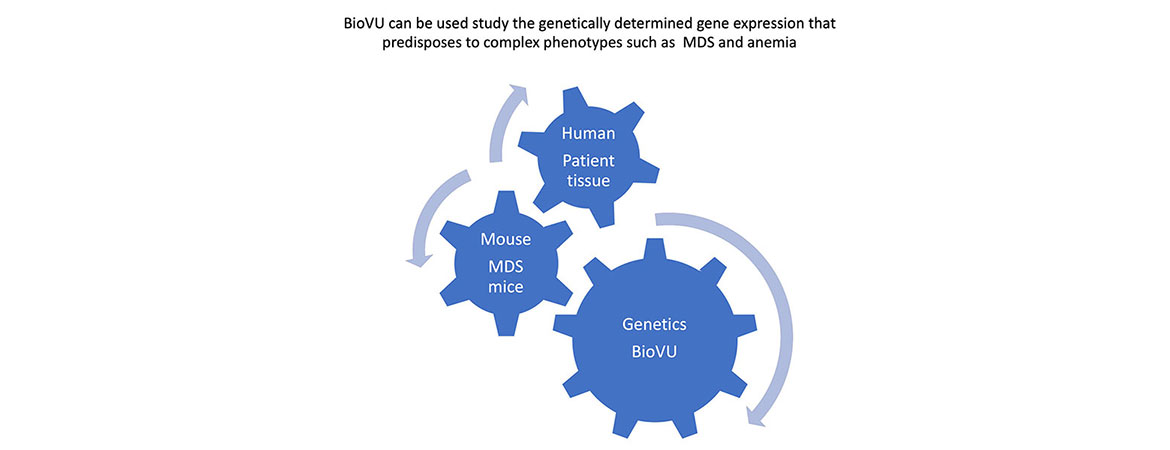
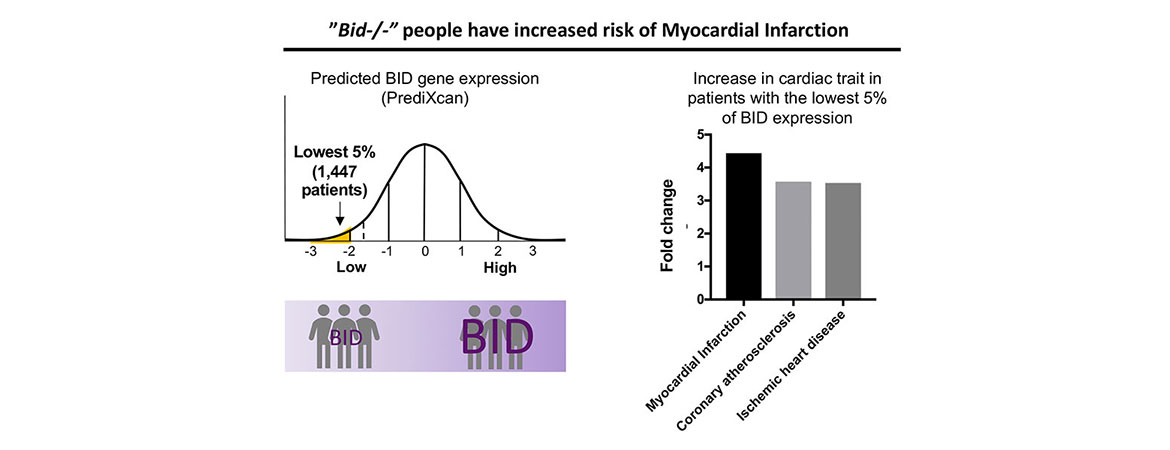
We are also interested in uncovering how genetically determined changes in expression of programmed cell death genes impacts susceptibility to human disease. We have utilized an integrative approach, leveraging mouse models as well as BioVU, to probe how genetically determined variations in gene expression can influence susceptibility to diseases such as myocardial infarction as well as bone marrow failure and leukemia.
The projects in my lab use hematopoietic cell culture systems, mouse models, immunofluorescence, electron microscopy, as well as flow cytometry and cell death assays to understand the signals and protein interactions that direct hematopoietic cells to die by apoptosis or necrosis. In addition, we use our mouse models to determine the effects of inhibiting necrosis on bone marrow failure and transformation to leukemia. Our studies provide new insights into the interplay between apoptosis and necrosis, and their role in hematopoiesis, bone marrow failure, and leukemogenesis. An additional focus is to determine the impact of genetically determined alterations in cell death pathways on susceptibility to human disease, using BioVU, in collaboration with Dr. Eric Gamazon
Postdoctoral position available: We have a postdoctoral fellow position open to study the impact of inflammatory cell death on hematopoiesis, and progression to bone marrow failure and leukemia