Welcome to the Zinkel Lab
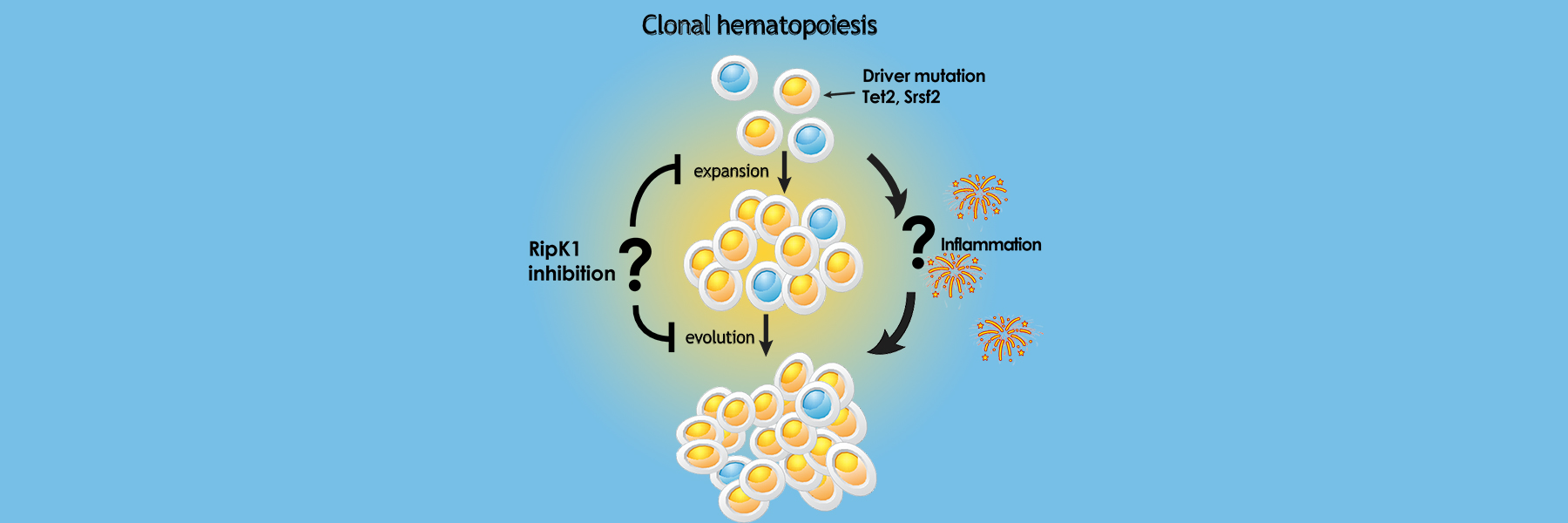
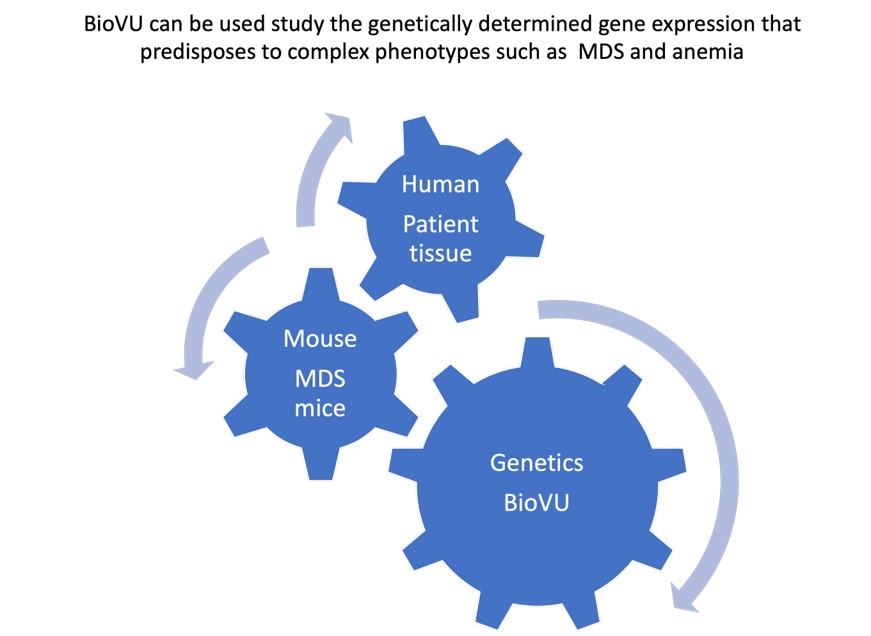
Our lab studies how inflammation in the bone marrow microenvironment impacts blood diseases such as clonal hematopoiesis, bone marrow failure (myelodysplastic syndrome (MDS) and leukemia and how inhibiting inflammation impacts disease.We use single cell analysis of mouse models and human samples as well as studies in BioVU (in collaboration with Eric Gamazon) to determine gene pathways that predispose to progression to disease.
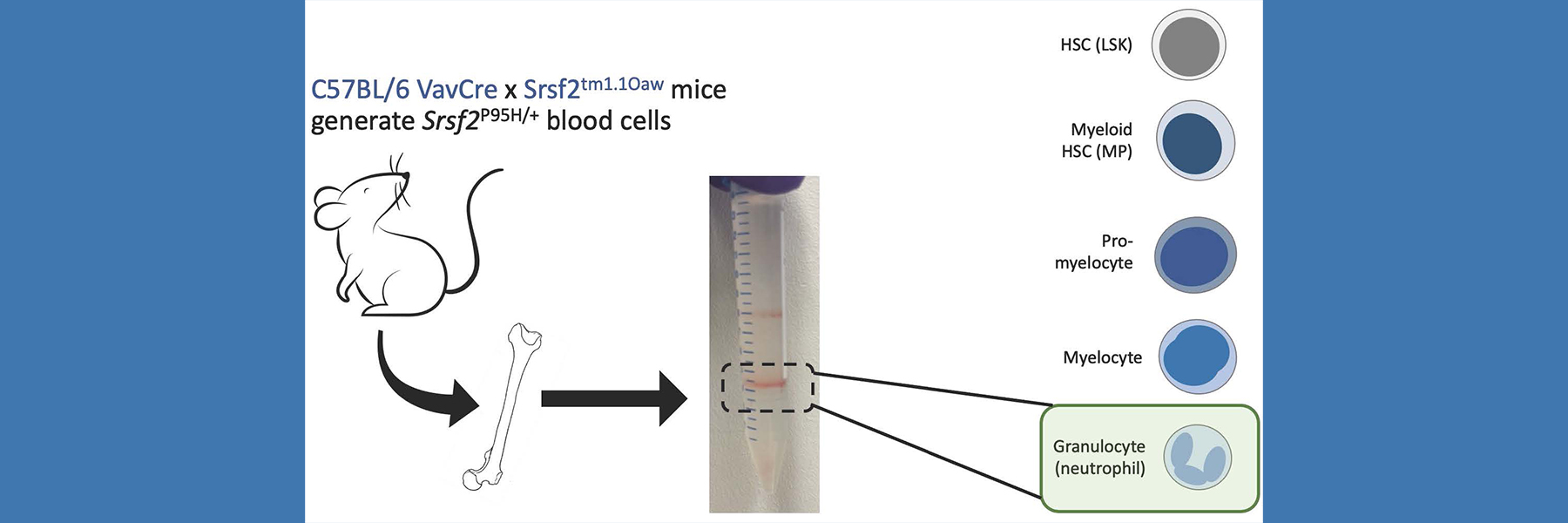
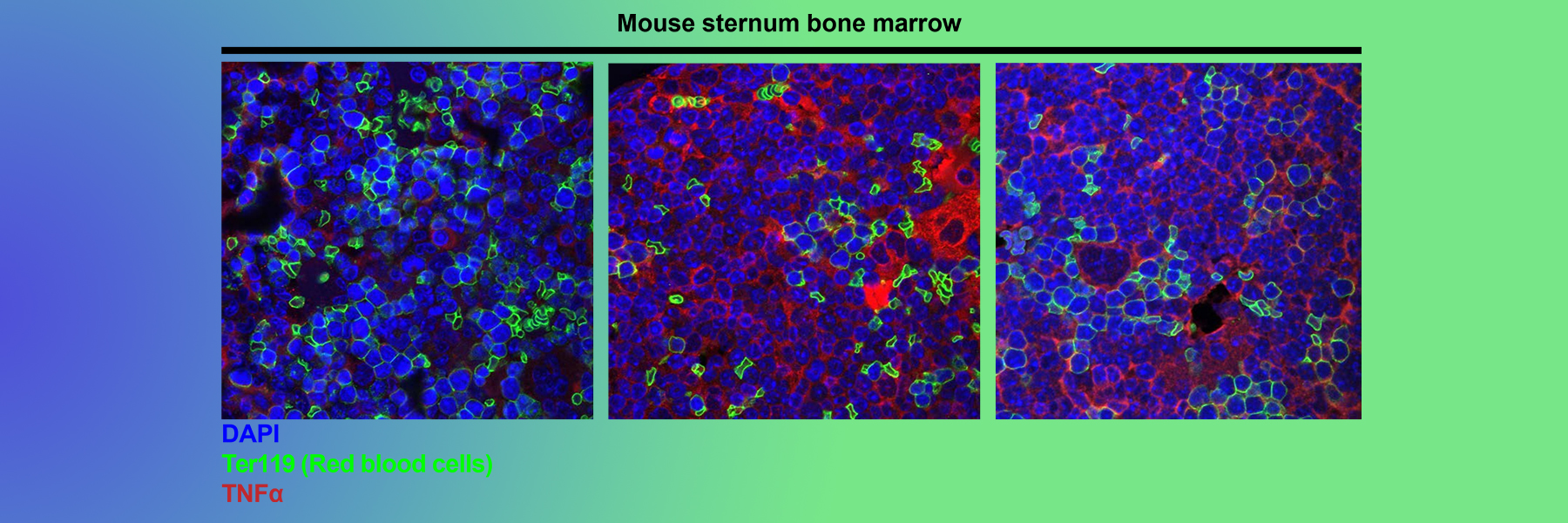
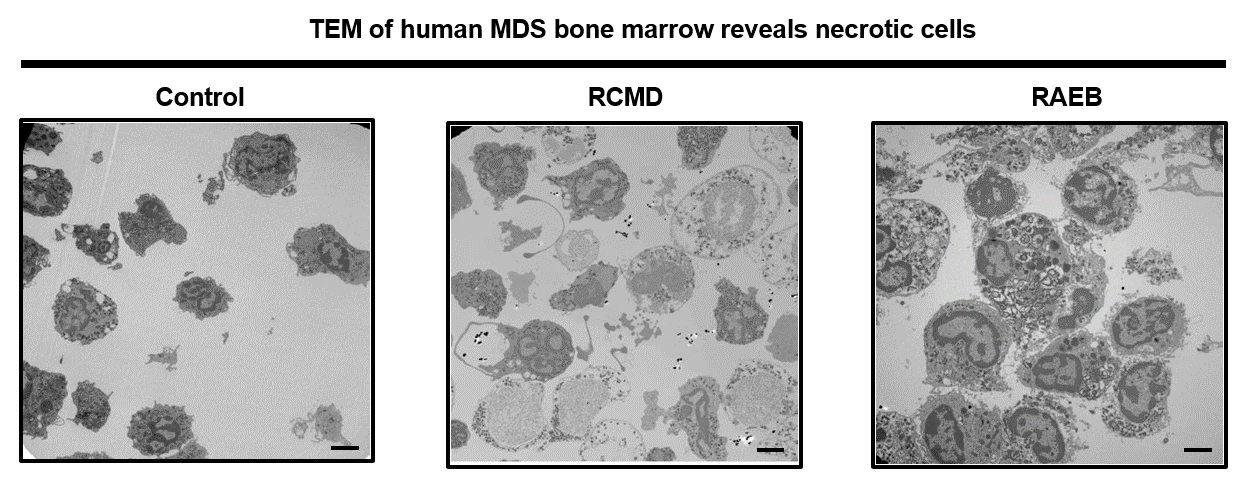
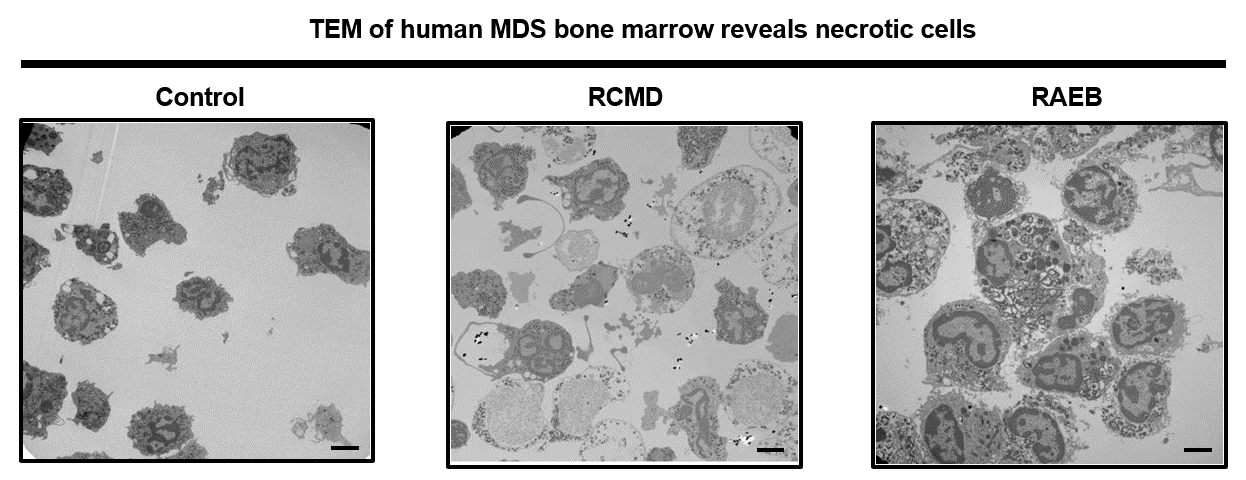
Current studies focus on how programmed cell death regulates homeostasis in the hematopoietic (blood) system. We have found that unrestrained programmed necrosis leads to bone marrow failure in mice that closely resembles the human disease Myelodysplastic syndrome (MDS) and find increased necrosis in human MDS bone marrow. Genetically inhibiting RIPK1 improves bone marrow inflammation in a mouse model of clonal hematopoiesis.
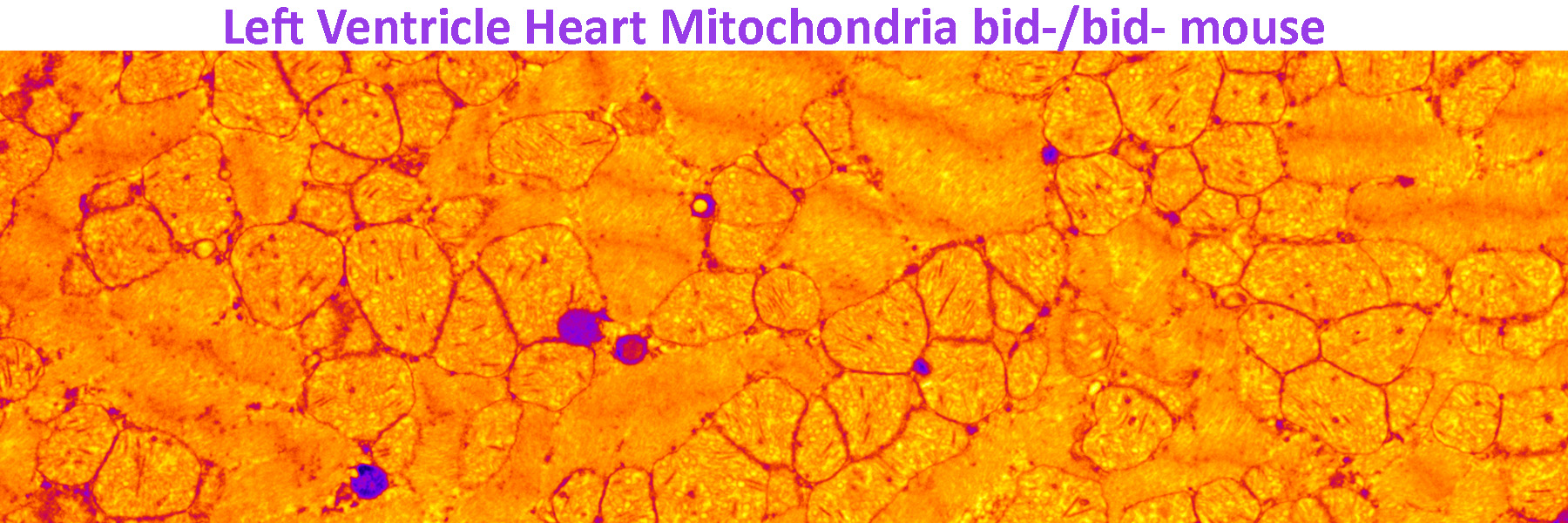
We are also interested in uncovering how genetically determined changes in expression of programmed cell death genes impacts susceptibility to human disease. We have utilized an integrative approach, leveraging mouse models as well as BioVU, to probe how genetically determined variations in gene expression can influence susceptibility to diseases such as myocardial infarction as well as bone marrow failure and leukemia. In addition, we use our mouse models to determine the effects of inhibiting necrosis on bone marrow failure and transformation to leukemia as well as to validate the findings from our BioVU studies.
Our studies provide new insights into the role of inflammatory cell death on the microenvironment and its impact on hematopoiesis, bone marrow failure, and leukemogenesis. An additional focus is to determine the impact of genetically determined alterations in cell death pathways on susceptibility to human disease, using BioVU, in collaboration with Dr. Eric Gamazon.
.