
The First Double-Blind
Randomized Study of SJS/TEN
NATIENS: North American Therapeutics in Epidermal Necrolysis Syndrome
About this study
The North American Therapeutics in Epidermal Necrolysis Syndrome (NATIENS) study is a multicenter, double-blind, randomized controlled assessment of three arms – two of systemic immunomodulatory therapies (cyclosporine and etanercept) and one of supportive care deemed to be the current standard of care.
Stevens-Johnson syndrome (SJS) and toxic epidermal necrolysis (TEN) are severe, life-threatening, immunologically mediated adverse drug reactions representing the same disease across a spectrum of severity that affect 60,000 patients per year globally at an approximate incidence of five cases per 1,000,000 in the United States.
The current evidence base for the management of SJS/TEN is limited, and there is no “standard of care” for treatment, as there are no double-blind randomized controlled trials for any treatment interventions, including supportive care. The scientific premise of our study is to address the critical knowledge gaps that currently limit the prevention, early diagnosis, and optimal care for patients with SJS/TEN.
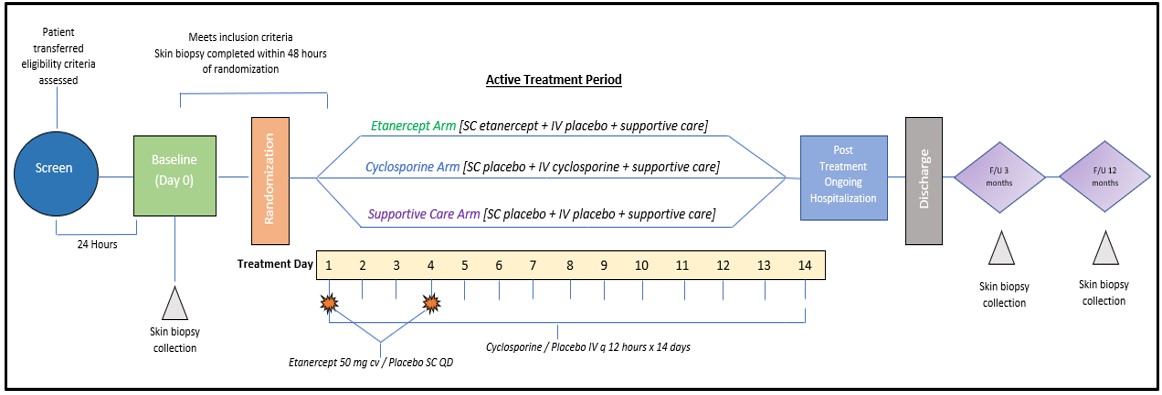
How it works
We are leveraging the opportunity of this controlled design to collect multiple patient samples, with the aim to discover new genetic and biological markers for the prevention and early diagnosis of SJS/TEN and, further, to define cellular and molecular mechanisms to facilitate the discovery of promising treatment strategies for SJS/TEN.
Samples collected during the study include DNA, PBMCs, RNA, plasma, serum, blister fluid cells and supernatant, sloughed epidermis, and punch biopsies of the skin. Samples will be bio-banked for immediate and targeted high-resolution HLA sequencing, cytokine profiling, and single-cell multidimensional analyses to identify biological markers that can lead to predicting the risks and outcomes of SJS/TEN.
Study goals
This study is the first to examine in a blinded, randomized, controlled design both the management and mechanisms of SJS/TEN. We anticipate these findings will lead to new ways to prevent, diagnose, and treat SJS/TEN and create a roadmap and evidence base for future studies in severe immunologically-mediated adverse drug reactions and other immunologically related diseases.
Questions?
- Learn more about the NATIENS study

CDSI clinical coordinator Madeline Marks conducts a training for research personnel at a study site for NATIENS.
