Featured CDSI Research of Dr. Cosby Stone
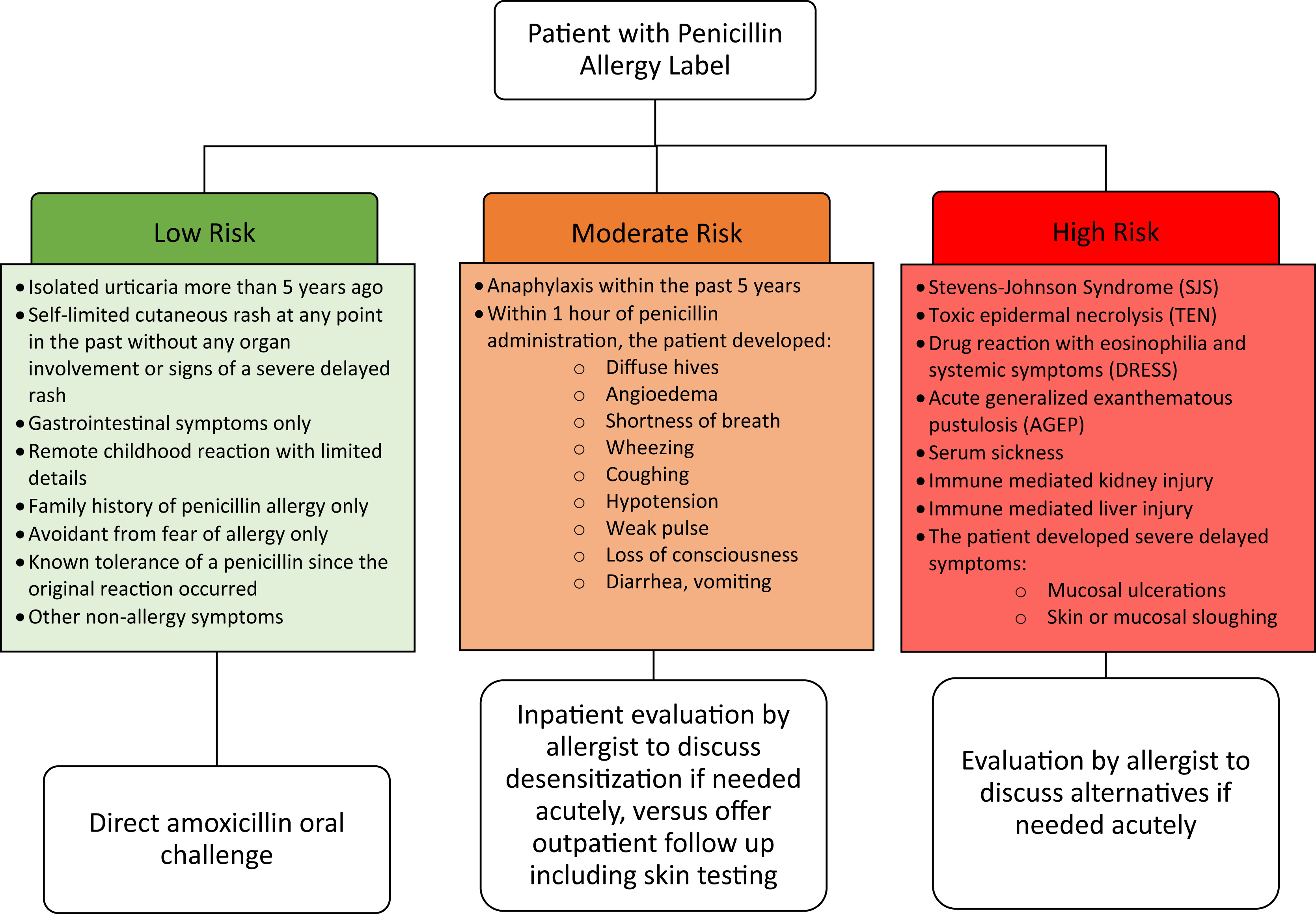
Dr. Cosby Stone focuses on the “right amount of testing,” which has involved identifying and delabeling low-risk penicillin allergies in the hospital and clinic. His efforts have saved lives and allowed patients to receive the “right drug at the right time.”

Cosby Allen Stone, Jr., MD, MPH, is an Assistant Professor of Medicine in the Division of Allergy, Pulmonology, and Critical Care Medicine within the Department of Medicine at Vanderbilt University Medical Center.
Expertise:
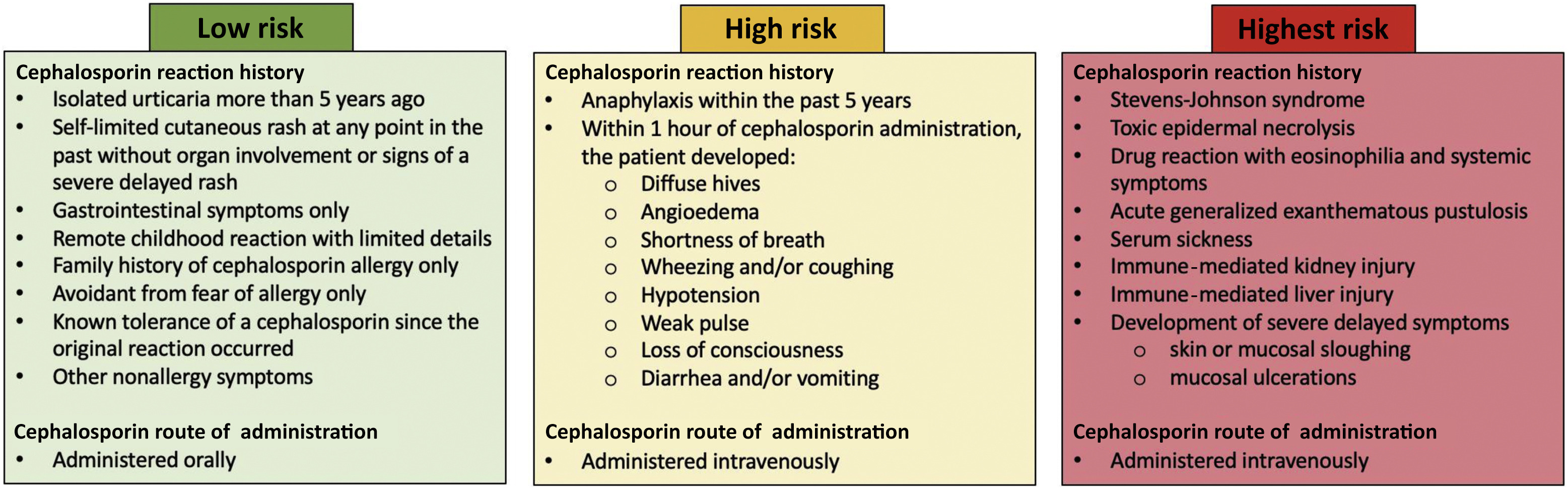
Dr. Stone has expertise in epidemiology, clinical drug allergy, and translational mechanistic projects in drug and vaccine allergy. He is engaged in pioneering clinical/translational research on beta-lactam antibiotic allergies with a secondary interest in immediate excipient allergies to alpha-gal and polyethylene glycols/polysorbates.
Current Focus:
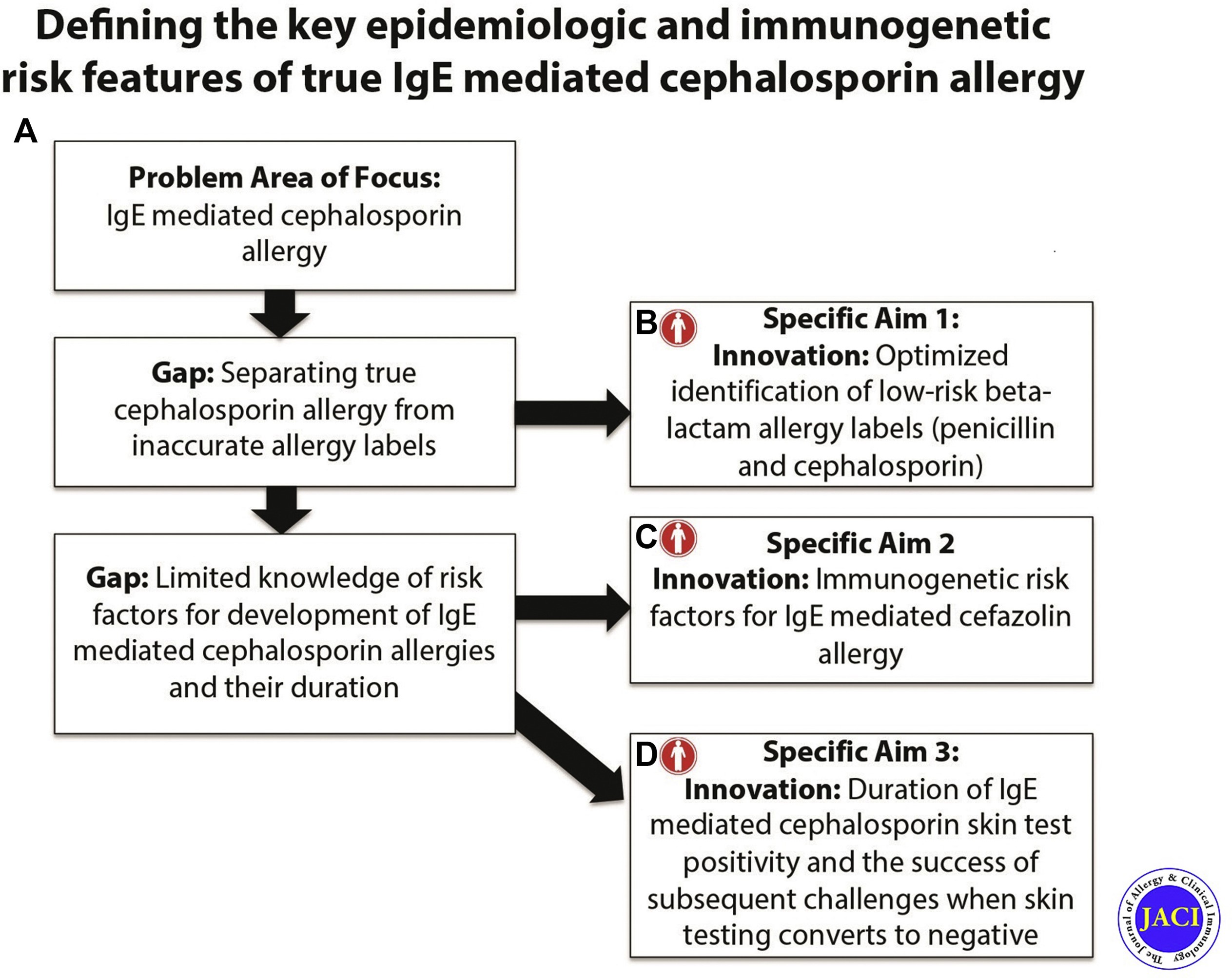
At CDSI, Dr. Stone’s work is currently culminating in a study on understanding and monitoring drug-induced anaphylaxis. Dr. Stone and CDSI researchers are collecting DNA information from individuals who have anaphylactic reactions from medications and/or foods to determine if certain genetic sequences are predisposed to certain reactions. This study, defined below, utilizes the knowledge and information Dr. Stone and his colleagues have developed through their research on beta-lactam allergies and their previous work identifying and delabeling low-risk penicillin allergies at VUMC.
Learn more about Dr. Stone's research projects below.