PALACE study
The use of penicillin allergy clinical decision rule to enable direct oral penicillin provocation, a multicenter randomized control trial
Study background
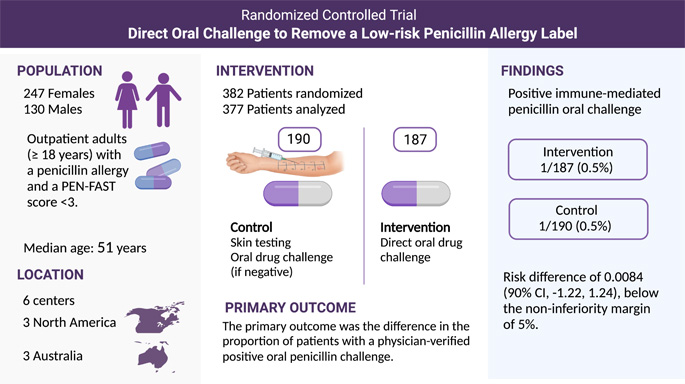
Penicillin allergy affects 1 in 10 Americans, more than 25 million in the United States. Seventy-five percent or more of people labeled with penicillin allergies receive this distinction by age three. Penicillin allergy labels remain even when there is confusion about whether the original symptoms may have been due to other causes, such as a viral rash. In the PALACE study, physician-scientists performed a simple test to determine if patients were still allergic to penicillin. Testing occurred at six sites across North America and Australia, including at the Center for Drug Safety and Immunology (CDSI).
Study design
Physician-scientists utilized a specialized risk assessment tool called PEN-FAST to assess patients' degree of penicillin allergy risk. Patients who reported what physicians consider a "low risk" penicillin allergy were eligible for enrollment in PALACE. Study staff randomized the 382 patients to either the standard of care or a direct oral challenge. The standard of care for a penicillin allergy assessment involves skin testing (prick testing and intradermal testing) and, if negative, a graded oral challenge (10% of the usual dose, and, if tolerated, followed by an intake of the standard amount). The second group went straight to oral challenge without a preceding skin test. The objective was to determine if the direct oral challenge was no worse than the standard of care among low-risk patients identified through PEN-FAST.
Study results
One patient randomized to the direct oral challenge and one patient randomized to the standard of care group experienced a positive reaction to the penicillin challenge. As a result, the total percentage of patients who experienced a reaction was 0.5%. There were no significant differences in adverse events between the standard of care and direct oral challenge, and there were no serious adverse events.
When interviewed by the VUMC News on the impact of these findings, CDSI director Dr. Elizabeth Phillips stated that "the evidence provided by the PALACE study will change clinical practice. Many patients in the United States do not have direct access to an allergist to provide specialized testing such as skin testing. Therefore, the ability to go to direct oral challenge with penicillin in low-risk patients, which can be carried out in any observed setting, will make it easier for patients in the United States to access health care to safely and effectively remove the label of penicillin allergy."
Principal investigators
- Ana Copaescu, MD, PhD, Researcher, McGill University
- Jason Trubiano, MBBS, PhD, Austin Hospital, Dohorty Institute, University of Melbourne
Read more