United States Drug Allergy Registry (USDAR)

About this study
Allergy specialists see patients to treat reported and documented allergies to various medications. Their evaluations often consist of a structured allergy history and, for some patients, drug allergy testing, which may include skin testing, patch testing, and low-dose oral administration of the related medication in patients determined to be low-risk.
The allergist's role is often to rule out allergies using these methods since true allergy represents only a fraction of those with reported drug allergies. However, given poor general documentation of drug allergy nationally in coding and billing and the electronic medical record, much about drug allergies still needs to be discovered.
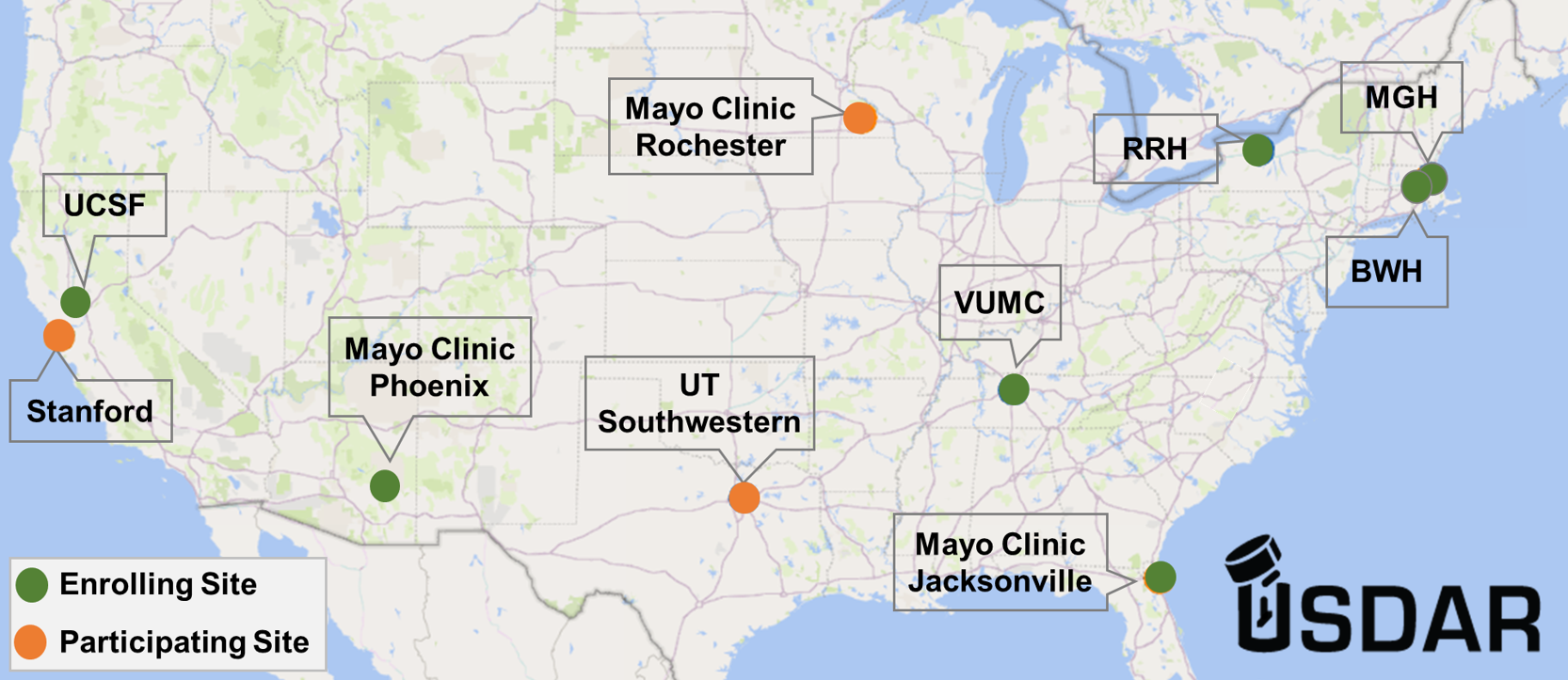
USDAR was founded as the first prospective, long-term community research project to address this gap in research. CDSI at Vanderbilt University Medical Center (VUMC) is one of seven sites, including the parent site, Massachusetts General Hospital, enrolling interested patients in this study. Through prospective standardized data collection and longitudinal assessments, participating scientists will advance patient care and research in drug allergy by identifying risk factors for true drug allergy and assessing the impact of allergy evaluation on the patient and their medical care.
Research objectives
Participating physician-scientists and their staff will utilize the deidentified data collected in this database as an informative tool. The information collected may enable them to modify and identify the best use for drug allergy evaluations, improve patient care, and alter clinic operations. Physician-scientists will also use these data in the aggregate to determine the phenotypes of patients with true drug hypersensitivity.
Study procedures
Study staff assess patient eligibility and obtain consent from interested patients during routine visits to the Vanderbilt Asthma, Sinus, and Allergy Program (VASAP) at Vanderbilt University Medical Center (VUMC). Consenting patients complete patient eligibility and quality of life questionnaires. Documentation of a drug allergy label is collected from patient health records, and administration of a drug allergy test may be performed by a clinician. Follow-up occurs through questionnaires sent via email to participants at six months, one, two, and three years. Follow-up also takes place with any referring providers at six months post-enrollment.
Data sources include:
- Patient self-report
- Referring provider survey
- Allergy notes
- Internal electronic health record review
- External record reviews (i.e., medical records from outside of VUMC)
- Pharmacy records
All collected data is compiled into a HIPAA-compliant database built and managed at Massachusetts General Hospital (MGH). A data use agreement is in place between VUMC and our research partners at MGH.
More information
- This study is led by Dr. Kim Blumenthal and Dr. Elizabeth Phillips is a co-investigator.
- Email drugsafetyresearch@vumc.org for more information
- USDAR-United States Drug Allergy Registry home