The Center for Drug Safety and Immunology is an internationally acclaimed center for excellence in research and clinical care.
Mission
The mission of The Center for Drug Safety and Immunology (CDSI) is to champion the growth of internationally recognized research in the areas of human immunology and drug safety and to promote advancement in the science and education of immunologically mediated adverse drug reactions (IM-ADR) that will lead to prevention, earlier diagnosis, and more effective treatment options as well as the improved safety of drugs across all patient populations.

About Us
The Center for Drug Safety and Immunology is unique in its scope and mission by merging human immunology, drug safety, and personalized medicine. CDSI also promotes the development and integration of multiple omic technologies.
We strive to understand mechanisms and risk of immune mediated adverse drug reactions. We aim to unite scientific and lay communities to improve the prevention, diagnosis and treatment of these IM-ADRs and ultimately the safety of drugs.
CDSI also promotes community engagement with patients and families affected by severe IM-ADR to enable increased awareness and support.
CDSI is
- A center of excellence in human immunology that operates under the broader umbrella of personalized medicine at Vanderbilt University Medical Center.
- Collaborating across Medicine, Dermatology, Pharmacology and Pathology, Microbiology and Immunology departments at VUMC.
- Physician scientists, scientists and research staff who work closely with community members to advance the understanding of genetic and mechanistic basis of immune-mediated adverse drug reaction (IM-ADR)
- Aiming to translate science into improved patient outcomes, safer design, development and use of drugs across patient populations
- Bringing together science with the community and developing approaches that lead to prediction, prevention, earlier diagnosis and more targeted treatment of IM-ADRs
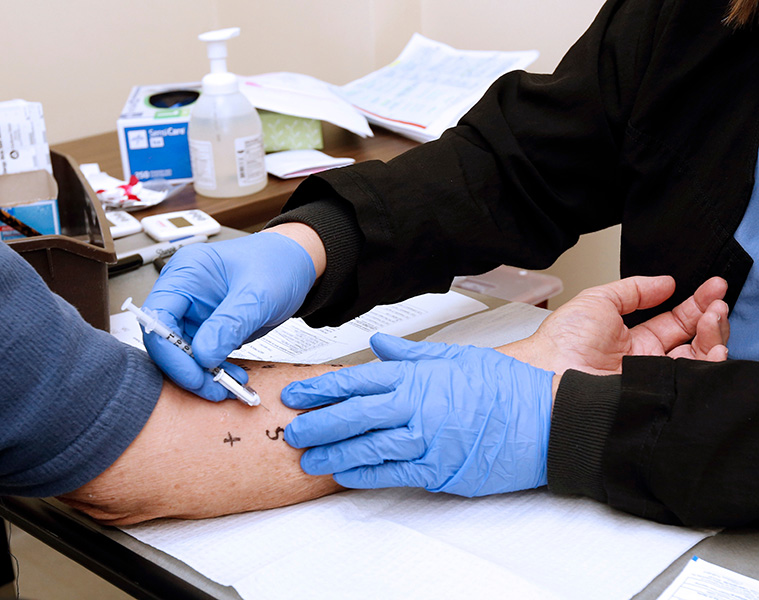
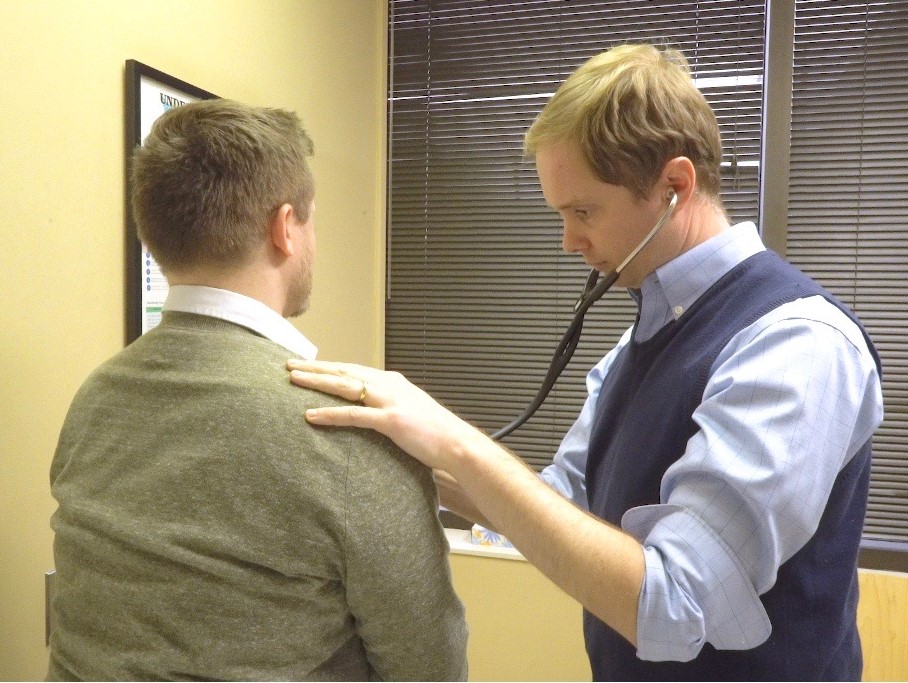
Clinical Care
We operate a Drug Safety Clinical Service in parallel to the research program and enroll patients into clinical and research studies.
This includes an outpatient clinic focusing on precision patient-centered care. Patients and their families who have experienced or been labeled as having an immune-mediated adverse drug reaction can receive:
- Genetic testing and counseling on IM-ADRs
- In vivo testing procedures that help appropriately label their ADR
- Expert advice on safe and effective future drug options
Specialized training
The Center for Drug Safety and Immunology fosters an outstanding research and clinical training environment with the prime purpose of developing the careers of future national and international leaders in the field of IM-ADRs.
The Center is currently the only one of its kind in North America and one of a few internationally that offers the depth and breadth of this cross-disciplinary and specialized training experience.
The outpatient clinic exemplifies the next generation of precision medicine where trainees learn important principles of immunology and pharmacology that translate into improved clinical care at all levels of practice and are not available in other training environments.

